��Ŀ����
��15�֣�ij�о�С���Լױ�Ϊ��Ҫԭ�ϣ���������·�ߺϳ�ҽҩ�м���F��Y��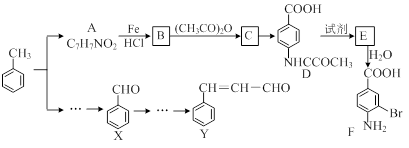
��֪����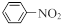

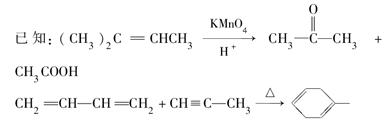
��2CH3CHO 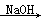 CH3CH(OH)CH2CHO
CH3CH(OH)CH2CHO  CH3CH��CHCHO
CH3CH��CHCHO
��ش��������⣺
�������й�F��˵����ȷ������ ����
| A������ʽ��C7H7NO2Br |
| B��������� |
| C���ܷ���ȡ����Ӧ�����۷�Ӧ |
| D��1 mol�� F�����Ժ�2 mol NaOH��Ӧ |
�ںϳ�F�Ĺ����У�B��C���費��ʡ�ԣ��������� ����
��D��E��Ӧ������Լ��� �� ����
��д��ͬʱ��������������A��ͬ���칹��Ľṹ��ʽ��д��3������
�ٱ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�� �ڷ����к��� ��CHO
�� ��
����X����ϩΪԭ�Ͽɺϳ�Y������ƺϳ�·�ߣ����Լ����ܼ���ѡ����
ע���ϳ�·�ߵ���д��ʽ��������ʾ������ͼ��
CH3CHO
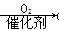 CH3COOH
CH3COOH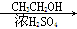 CH3COOCH2CH3
CH3COOCH2CH3
��B��C ��������Ӧ
��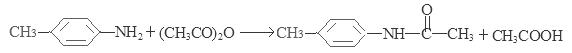
�����ױ���������������Ӧ֮ǰ���ȱ�������
��Br2 / FeBr3 ��Br2 / Fe
��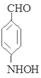 ��
��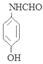 ��
��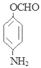 ��
��
��H2C��CH2 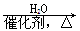 CH3CH2OH
CH3CH2OH 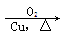 CH3CHO
CH3CHO 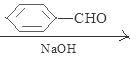


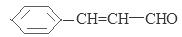 ��
��
����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
������6���л�����ṩ���Լ��ֱ���𣬽������Լ��������������������ڸ��������ϡ�
(1)________��(2)________��(3)________��(4)________��
(5)________��(6)________��
| �л��� | �Լ� | ���� |
| (1)�ױ� (2)����ϩ (3)���� (4)������ (5)���� (6)������ | a.��ˮ b.���Ը��������Һ c.Ũ���� d.��ˮ e.���Ȼ�����Һ f.����������ͭ | A.��ɫ��ȥ B.��ɫ��ȥ C.����ɫ D.���ֺ�ɫ ���� E.�ʻ�ɫ F.����ɫ |
 ����
����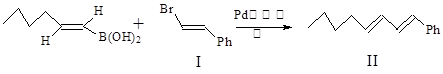
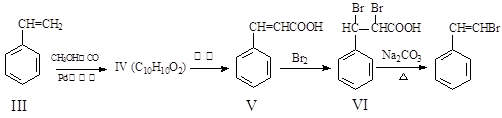
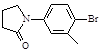 ���뻯���
���뻯���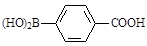 ���ܷ���ż����Ӧ����һ�ֿ�����ҩ�д���ÿ�����ҩ��Ľṹ��ʽ ��
���ܷ���ż����Ӧ����һ�ֿ�����ҩ�д���ÿ�����ҩ��Ľṹ��ʽ ��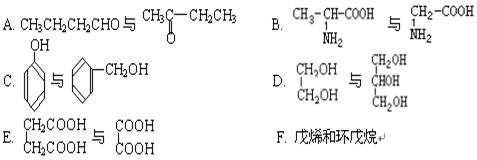
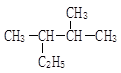
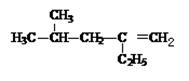

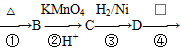 PHB
PHB