��Ŀ����
1��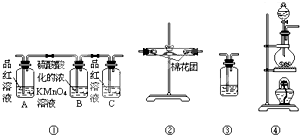 ��֪��SO2�����������ữ��ŨKMnO4��Һ��Ӧ��Ҳ����ʹ�����ʯ��ˮ����ǣ���ͼ����Ũ������ľ̿���ڼ��������·�����Ӧ����CO2��SO2��H2O��
��֪��SO2�����������ữ��ŨKMnO4��Һ��Ӧ��Ҳ����ʹ�����ʯ��ˮ����ǣ���ͼ����Ũ������ľ̿���ڼ��������·�����Ӧ����CO2��SO2��H2O����1���ڼ��������£�Ũ������ľ̿�۷�Ӧ�Ļ�ѧ����ʽ�ǣ�2H2SO4��Ũ��+C$\frac{\underline{\;\;��\;\;}}{\;}$2SO2��+CO2��+2H2O��
��2��������ͼ���и�װ�����һ��ʵ�飬��֤Ũ������ľ̿�۷�Ӧ�IJ����Щװ�õ�����˳��ܡ��ڡ��١��ۣ�
�������������������ҵķ���
��3��Bƿ��Һ������������SO2���壮��ʵ������˵�������������Ư���Ե�ʵ��������A��Ʒ����Һ��ɫ��
��4��װ�â�����ʢ����ˮ����ͭ���������ɰ�ɫ��Ϊ��ɫ��
���� ��1��ľ̿����Ũ���ᷢ����Ӧ����SO2��CO2���壻
��2�����������̼�Ͷ�������ʱ�õ�����Һ�о�����ˮ�������ȼ���ˮ�Ĵ��ڣ�������̼�Ͷ������������ʹ����ʯ��ˮ����ǣ������ȼ�����������ٳ�ȥ�������������̼��
��3������ʵ��Ŀ�ĺ�װ��ͼ���Dz���������Լ������ü�������Ӧ�������忼�ǣ�AΪ�����������װ�ã�B��CΪ��ȥ�����������Ƿ�������������װ�ã���Ʒ����Һ��ɫ��֤����������к��ж�������
��4����ˮ����ͭΪ��ɫ���壬��ˮ�ɱ�Ϊ��ˮ����ͭ��CuSO4•5H2O��Ϊ��ɫ���壮
��� �⣺��1����ľ̿����Ũ���ᷢ����Ӧ����SO2��CO2���壬��Ӧ�ķ���ʽΪ2H2SO4��Ũ��+C$\frac{\underline{\;\;��\;\;}}{\;}$2SO2��+CO2��+2H2O��
�ʴ�Ϊ��2H2SO4��Ũ��+C$\frac{\underline{\;\;��\;\;}}{\;}$2SO2��+CO2��+2H2O��
��2�����������̼�Ͷ�������ʱ�õ�����Һ�о�����ˮ�������ȼ���ˮ�Ĵ��ڣ�������̼�Ͷ������������ʹ����ʯ��ˮ����ǣ������ȼ�����������ٳ�ȥ�������������̼��˳��Ϊ���ܡ��ڡ��١��ۣ�
�ʴ�Ϊ���ܢڢ٢ۣ�
��3��AΪ�����������װ�ã�B��CΪ��ȥ�����������Ƿ�������������װ�ã������������Ư���ԣ���A��Ʒ����Һ��ɫ����֤�����ڶ����������壬
�ʴ�Ϊ������SO2���壻A��Ʒ����Һ��ɫ��
��4����ˮ����ͭΪ��ɫ���壬��ˮ�ɱ�Ϊ��ˮ����ͭ��CuSO4•5H2O��Ϊ��ɫ���壬�Ӷ�֤��ˮ�Ĵ��ڣ�
�ʴ�Ϊ���ɰ�ɫ��Ϊ��ɫ��
���� ���⿼��Ũ����Ļ�ѧ���ʣ���Ŀ�Ѷ��еȣ���ȷ����������õ��Լ��Լ�������Ⱥ�˳��Ϊ���ؼ��������ۺ��Խ�ǿ����ֿ�����ѧ���ķ�����������������ѧʵ��������

| A�� | NC13��N-C1��������CCl4��C-C1�������� | |
| B�� | NC13�����е�����ԭ�Ӿ��ﵽ8�����ȶ��ṹ | |
| C�� | NCl3�����Ǽ��Է��� | |
| D�� | NBr3�ķе��NCl3�ķе�� |
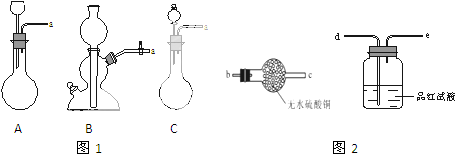

 ijС���Է���м��ϡ���ᡢ���ͣ�NH4��2SO4��ҺΪԭ�ϣ�����һϵ�з�Ӧ�Ͳ����ϳ���dz����ɫ����X��Ϊȷ������ɣ���������ʵ�飮
ijС���Է���м��ϡ���ᡢ���ͣ�NH4��2SO4��ҺΪԭ�ϣ�����һϵ�з�Ӧ�Ͳ����ϳ���dz����ɫ����X��Ϊȷ������ɣ���������ʵ�飮