��Ŀ����
����������Ag2O2������п���Ե�������Ļ������ʣ���ͨ�����з�Ӧ�Ʊ���
��K2S2O8+2AgNO3+4KOH�TAg2O2��+2KNO3+2K2SO4+2H2O��
��1����֪Ag��ԭ������Ϊ47��д��Ag��̬ԭ�ӵĺ�������Ų�ʽ______��Ag�����ڱ��е�λ��______��
��2���Ʊ�Ag2O2�ķ�Ӧ������ȫ��______��ϴ�ӡ�______�Ȳ��������ɵõ�Ag2O2���壬����Ag2O2�Ƿ�ϴ�Ӹɾ��ķ�����______��
��3��һ����п��Ag2O2��Zn�����Ե�صĵ������ҺΪKOH��Һ����طŵ�ʱ������Ag���ɣ������л�����A��ֻ����Zn��K��O�����ɣ�������A�У��ء�п����Ԫ�ص�������Ϊ78��65����õ�ص��ܷ�Ӧ����ʽΪ______��
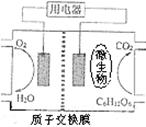
��4������п��ص������[CO��NH2��2]�ļ�����Һ����ȡH2����װ����ͼ��ʾ�������и�Ĥ����ֹ����ͨ����M��N��Ϊ���Ե缫����
��N������п��ص�______���Zn����Ag2O2������������
��M���ĵ缫��ӦʽΪ______��

��K2S2O8+2AgNO3+4KOH�TAg2O2��+2KNO3+2K2SO4+2H2O��
��1����֪Ag��ԭ������Ϊ47��д��Ag��̬ԭ�ӵĺ�������Ų�ʽ______��Ag�����ڱ��е�λ��______��
��2���Ʊ�Ag2O2�ķ�Ӧ������ȫ��______��ϴ�ӡ�______�Ȳ��������ɵõ�Ag2O2���壬����Ag2O2�Ƿ�ϴ�Ӹɾ��ķ�����______��
��3��һ����п��Ag2O2��Zn�����Ե�صĵ������ҺΪKOH��Һ����طŵ�ʱ������Ag���ɣ������л�����A��ֻ����Zn��K��O�����ɣ�������A�У��ء�п����Ԫ�ص�������Ϊ78��65����õ�ص��ܷ�Ӧ����ʽΪ______��
��4������п��ص������[CO��NH2��2]�ļ�����Һ����ȡH2����װ����ͼ��ʾ�������и�Ĥ����ֹ����ͨ����M��N��Ϊ���Ե缫����
��N������п��ص�______���Zn����Ag2O2������������
��M���ĵ缫��ӦʽΪ______��

��1��Ԫ�ص���������֪������Ϊ47����������Ӳ������Ϊ2��8��18��18��1����[Kr]4d105S1��1S22S22P63S23P63d104S24P64d105S1�����Ӳ�����������������������������������������λ�ڵ�������IB�壬
�ʴ�Ϊ��[Kr]4d105S1��1S22S22P63S23P63d104S24P64d105S1����������IB�壻
��2�������Һ��ķ�����ù��˵ķ�����Ȼ����ϴ�Ӹ�����ɵõ��������ʣ������Ƿ�ϴ����ȫ����ȡ���һ����Һ��������Һ���Ƿ���SO42-������1��2��BaCl2��Һ���������ְ�ɫ���ǣ���ʾ��ϴ����ȫ��
�ʴ�Ϊ�����ˡ����ȡ���һ��ϴ��Һ���������뼸��BaCl2��Һ�����а�ɫ���Dz�����˵��δϴ�Ӹɾ�����������˵����ϴ�Ӹɾ���
��3����طŵ�ʱ������Ag2O2ת��ΪAg��������A�У��ء�п����Ԫ�ص�������Ϊ78��65��������Aֻ����Zn��K��O��ԭ�Ӹ���֮�ȵ��ڻ�ѧʽ��ԭ����֮�ȣ����Ը�����Znת��ΪK2ZnO2����Ӧ��Ӧ��KOH�μӣ��÷�Ӧ�ķ���ʽΪAg2O2+2Zn+4KOH+2H2O=2K2Zn��OH��4+2Ag��
�ʴ�Ϊ��Ag2O2+2Zn+4KOH=2Ag+2K2ZnO2+2H2O��
��4���ٸ���װ���еIJ��֪���õ�ط�Ӧʱ�У���Ԫ�ػ��ϼ���-3�۱�Ϊ0�ۣ�HԪ�ػ��ϼ���+1�۱�Ϊ0�ۣ���Ԫ�ر���������Ԫ�ر���ԭ���������ɵ����ĵ缫M�����������������ĵ缫N����������п����У������ǽ���п�������Ǻ���п�������ļ�����N������п��ص�п��������
�ʴ�Ϊ��Zn��
��M���������ü�����ʧ���ӵ�������Ӧ���ü��ϵĵ缫��ӦʽΪ��CO��NH2��2-6e-+80H-=N2��+CO32-+6H2O��
�ʴ�Ϊ��CO��NH2��2-6e-+80H-=N2��+CO32-+6H2O��
�ʴ�Ϊ��[Kr]4d105S1��1S22S22P63S23P63d104S24P64d105S1����������IB�壻
��2�������Һ��ķ�����ù��˵ķ�����Ȼ����ϴ�Ӹ�����ɵõ��������ʣ������Ƿ�ϴ����ȫ����ȡ���һ����Һ��������Һ���Ƿ���SO42-������1��2��BaCl2��Һ���������ְ�ɫ���ǣ���ʾ��ϴ����ȫ��
�ʴ�Ϊ�����ˡ����ȡ���һ��ϴ��Һ���������뼸��BaCl2��Һ�����а�ɫ���Dz�����˵��δϴ�Ӹɾ�����������˵����ϴ�Ӹɾ���
��3����طŵ�ʱ������Ag2O2ת��ΪAg��������A�У��ء�п����Ԫ�ص�������Ϊ78��65��������Aֻ����Zn��K��O��ԭ�Ӹ���֮�ȵ��ڻ�ѧʽ��ԭ����֮�ȣ����Ը�����Znת��ΪK2ZnO2����Ӧ��Ӧ��KOH�μӣ��÷�Ӧ�ķ���ʽΪAg2O2+2Zn+4KOH+2H2O=2K2Zn��OH��4+2Ag��
�ʴ�Ϊ��Ag2O2+2Zn+4KOH=2Ag+2K2ZnO2+2H2O��
��4���ٸ���װ���еIJ��֪���õ�ط�Ӧʱ�У���Ԫ�ػ��ϼ���-3�۱�Ϊ0�ۣ�HԪ�ػ��ϼ���+1�۱�Ϊ0�ۣ���Ԫ�ر���������Ԫ�ر���ԭ���������ɵ����ĵ缫M�����������������ĵ缫N����������п����У������ǽ���п�������Ǻ���п�������ļ�����N������п��ص�п��������
�ʴ�Ϊ��Zn��
��M���������ü�����ʧ���ӵ�������Ӧ���ü��ϵĵ缫��ӦʽΪ��CO��NH2��2-6e-+80H-=N2��+CO32-+6H2O��
�ʴ�Ϊ��CO��NH2��2-6e-+80H-=N2��+CO32-+6H2O��

��ϰ��ϵ�д�
�����Ŀ
 2PbSO4��2H2O ����������ȷ���ǣ� ��
2PbSO4��2H2O ����������ȷ���ǣ� ��