��Ŀ����
��10�֣���һƿ������Һ�����п��ܺ���K+��Na+��Mg+��Al3+��Cl-��AlO2-��I-��OH���еļ��֣�ȡ����Һ��������ʵ�飻
����pH��ֽ��⣬��Һ��ǿ���ԣ���ɫ��Ӧ����ɫ�ܲ����۲쵽��ɫ���档
��ȡ������Һ����������CCl4���������Ƶ���ˮ���������ú�CCl4����Ϻ�ɫ��
����ȡ������Һ����μ���ϡHCl��Һ��ʹ��Һ�Ӽ�����Ϊ���ԣ�����ӹ�������Һ���ֳ������μ���Ϻ���Һ�г�����ʧ��
��������ʵ����ʵ������������⣺
(1)����Һ�п϶����ڵ�������____ ___ ��
(2)����Һ�п϶������ڵ�������___ ____ ��
(3)����Һ�л�����ȷ���Ƿ���ڵ�������___ ____��
����pH��ֽ��⣬��Һ��ǿ���ԣ���ɫ��Ӧ����ɫ�ܲ����۲쵽��ɫ���档
��ȡ������Һ����������CCl4���������Ƶ���ˮ���������ú�CCl4����Ϻ�ɫ��
����ȡ������Һ����μ���ϡHCl��Һ��ʹ��Һ�Ӽ�����Ϊ���ԣ�����ӹ�������Һ���ֳ������μ���Ϻ���Һ�г�����ʧ��
��������ʵ����ʵ������������⣺
(1)����Һ�п϶����ڵ�������____ ___ ��
(2)����Һ�п϶������ڵ�������___ ____ ��
(3)����Һ�л�����ȷ���Ƿ���ڵ�������___ ____��
(1)K+��I-��AlO2-��OH - (2)Mg2+ Al3+ (3)Na+��Cl-
�������⣬�ɢ�֪����K+��OH�C����Mg2+ Al3+���ɢ�֪����I-���ɢ�֪����AlO2-��Na+��Cl-��ȷ����

��ϰ��ϵ�д�
 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
�����Ŀ
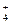 ��Fe2+��I
��Fe2+��I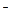 ��Br
��Br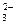 ��SO
��SO