��Ŀ����
2����������ʯ����Ҫ�ɷ֣�K2SO4•Al2��SO4��3•2Al2O3•6H2O����������Fe2O3���Ʊ�����������������ͼ��ʾ��
��1������¯�з�ӦΪ��2Al2��SO4��3+3S�T2Al2O3+9SO2�� �÷�Ӧ���������ǣ�Al2��SO4��3��������1mol Al2O3����ת�Ƶ�������3.612��1024����
��2�������ܽ�ʱ�����ӷ���ʽ��Al2O3+2OH-=2AlO2-+H2O��
��3����������к���Fe2O3������Լ���HCl��KSCN��
��4��ĸҺ�����ʵ���Ҫ�ɷֵĻ�ѧʽΪ��K2SO4��Na2SO4����Һ����pH���ˡ�ϴ�ӿɵ�Al��OH��3������֤������ϴ�Ӹɾ���ʵ������������ǣ�ȡ���һ�ε�ϴ��Һ���Թ��У�����BaCl2��Һ���ް�ɫ����������֤��ϴ�Ӹɾ���
���� ���Ʊ����̿�֪������ʯ��ˮ���ڱ���¯�з�����Ӧ�Ļ�ѧ����ʽΪ2Al2��SO4��3+3S$\frac{\underline{\;����\;}}{\;}$2Al2O3+9SO2���õ���¯����ҪΪSO2�������к�K2SO4��Al2O3��������Fe2O3��Ȼ���NaOH��H2O�ܽ�ʱ������Al2O3+2OH-=2AlO2-+H2O�������к�Fe2O3����Һ�к�K2SO4��NaAlO2��Na2SO4��������������pH��AlO2-ת��ΪAl��OH��3��ĸҺ��������Ҫ��K+��Na+��SO42-����������ΪK2SO4��Na2SO4���Դ������
��� �⣺���Ʊ����̿�֪������ʯ��ˮ���ڱ���¯�з�����Ӧ�Ļ�ѧ����ʽΪ2Al2��SO4��3+3S$\frac{\underline{\;����\;}}{\;}$2Al2O3+9SO2���õ���¯����ҪΪSO2�������к�K2SO4��Al2O3��������Fe2O3��Ȼ���NaOH��H2O�ܽ�ʱ������Al2O3+2OH-=2AlO2-+H2O�������к�Fe2O3����Һ�к�K2SO4��NaAlO2��Na2SO4��������������pH��AlO2-ת��ΪAl��OH��3��ĸҺ��������Ҫ��K+��Na+��SO42-����������ΪK2SO4��Na2SO4��
��1����Ӧ��Al2��SO4��3��SO2����Ԫ�ػ��ϼ���+6�۽���Ϊ+4�ۣ���Al2��SO4��3������������Ӧ����������Ԫ�ػ��ϼ���0������ΪSO2��+4�ۣ�����Ϊ��ԭ��������1molAl2O3��Ҫ������ʵ���Ϊ1mol��$\frac{3}{2}$=1.5mol��ת�Ƶ��ӵ����ʵ���Ϊ1.5mol��4=6mol��ת�Ƶ�����ĿΪ6mol��6.02��1023mol-1=3.612��1024��
�ʴ�Ϊ��Al2��SO4��3��3.612��1024��
��2���ɹ������̿�֪�������ܽ�Ϊ������������������Һ��Ӧ����ƫ�����ƣ����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��3���������ӵ���Һ��KSCN��Һ��ΪѪ��ɫ�ɼ��������ӣ�ѡ��HCl�ܽ���壬KSCN���������ӣ����������к���Fe2O3������Լ�ΪHCl��KSCN��
�ʴ�Ϊ��HCl��KSCN��
��4��������������֪���������pHֵʱ��AlO2-ת��ΪAl��OH��3��ĸҺ��������Ҫ��K+��Na+��SO42-����������ΪK2SO4��Na2SO4������ϴ�Ӹɾ�����治����������ӣ�����ϴ�Ӹɾ���ʵ�������������ȡ���һ�ε�ϴ��Һ���Թ��У�����BaCl2��Һ���ް�ɫ����������֤��ϴ�Ӹɾ���
�ʴ�Ϊ��K2SO4��Na2SO4��ȡ���һ�ε�ϴ��Һ���Թ��У�����BaCl2��Һ���ް�ɫ����������֤��ϴ�Ӹɾ���
���� ���⿼���Ʊ�ʵ�鷽������ƣ�Ϊ��Ƶ���㣬�����Ʊ������еķ�Ӧ�����������ᴿ����Ϊ���Ĺؼ���ע�����ӷ�Ӧ��������ԭ��Ӧ�����Ӽ�����ۺ�Ӧ�ã����ط�����ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

| A�� | 0.2mol•L-1 H2C2O4��Һ��C��H+����C��H2C2O4����C��H2C2O4-����C ��C2O42-�� | |
| B�� | �����£�pH=11��NaOH��Һ��pH=3�Ĵ�����Һ�������Ϻ�������Һ��PH��7 | |
| C�� | �ڣ�NH4��2SO4��Һ�У�C��NH4+��+C��NH3•H2O��=$\frac{1}{2}$C��SO42-�� | |
| D�� | �����ᣨHN3������������ƣ�0.1 mol•L-1NaN3��Һ��C ��N3-����C��Na+����C��OH-����C ��H+�� |
| A�� | �ӳɡ���ȥ��ȡ�� | B�� | ��ȥ���ӳɡ�ȡ�� | C�� | ȡ������ȥ���ӳ� | D�� | ��ȥ���ӳɡ���ȥ |
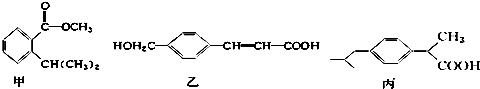
�����й�˵���д�����ǣ�������
| A�� | �ס��ҡ������Ƿ����廯���ֻ����������̼��������Һ��Ӧ | |
| B�� | ֻ��̼��������Һ��������Һ�ܼ���ס��ҡ��� | |
| C�� | ������������Ӧ����ͬ���ʵ����ļס��ҡ����������������ʵ���֮��Ϊ3��4��3 | |
| D�� | �Ļ�ѧʽΪC11H14O2���Һ������ֺ��������� |
| A�� | 11.2g | B�� | 8.4g | C�� | 16.8g | D�� | 22.4g |
 ��
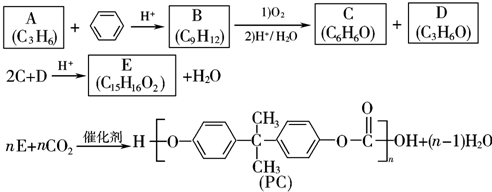
��
 ��
�� ��
�� ��д���ṹ��ʽ����
��д���ṹ��ʽ����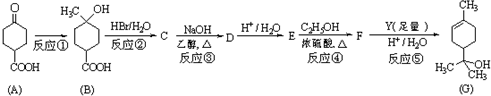
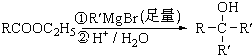
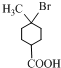 +2NaOH
+2NaOH
 +NaBr+2H2O��
+NaBr+2H2O��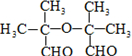 ��
�� H2��g��+CO2��g����H��0����������������������
H2��g��+CO2��g����H��0����������������������