ЬтФПФкШн
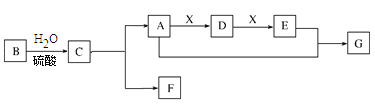
ЁОЬтФПЁПЖЬжмЦкжїзхдЊЫиAЁЂBЁЂCЁЂDЁЂEдзгађЪ§вРДЮдіДѓЃЛAгыBПЩаЮГЩ4КЫ10ЕчзгЕФЗжзгЃЛCЁЂEЭЌжїзхЃЌDЁЂEЭЌжмЦкЃЛCКЭDПЩаЮГЩРызгЛЏКЯЮяD2CЃЌD2CжавѕбєРызгЕФЕчзгВуНсЙЙЯрЭЌЃЌЧвЕчзгзмЪ§ЮЊ30ЁЃ
ЧыЛиД№ЯТСаЮЪЬт![]() гУдЊЫиЗћКХБэЪО
гУдЊЫиЗћКХБэЪО![]() ЃК
ЃК
ЃЈ1ЃЉдЊЫиAЕФвЛжжЭЌЮЛЫижЪзгЪ§гыжазгЪ§ЯрЕШЃЌетжжЭЌЮЛЫиЕФЗћКХЪЧ______ЁЃ
ЃЈ2ЃЉдЊЫиBдкжмЦкБэжаЕФЮЛжУЪЧ______ЁЃ
ЃЈ3ЃЉдЊЫиAгыBаЮГЩ10ЕчзгЕФЗжзгЃЌЦфЫЎШмвКГЪМюадЃЌгУЕчРыЗНГЬЪНБэЪО______ЁЃ
ЃЈ4ЃЉдЊЫиAЁЂBЁЂCаЮГЩЕФдзгИіЪ§БШЮЊ4ЃК2ЃК3ЕФбЮЃЌЪєгк______![]() ЬюЁАРызгЁАЛђЁАЙВМлЁА
ЬюЁАРызгЁАЛђЁАЙВМлЁА![]() ЛЏКЯЮяЃЌКЌгаЕФЛЏбЇМќРраЭЮЊ______ЁЃ
ЛЏКЯЮяЃЌКЌгаЕФЛЏбЇМќРраЭЮЊ______ЁЃ
ЃЈ5ЃЉдЊЫиCЁЂDаЮГЩЛЏКЯЮяD2C2ЕФЕчзгЪНЮЊ______ЃЌЦфгыЫЎЗДгІЕФРызгЗНГЬЪНЮЊ______ЁЃ
ЃЈ6ЃЉдЊЫиCЁЂDЁЂEЁЂFаЮГЩМђЕЅРызгЕФАыОЖгЩаЁЕНДѓЕФЫГађЮЊ______ЁЃ
ЃЈ7ЃЉдЊЫиEгыFЯрБШЃЌдЊЫидзгЕУЕчзгФмСІНЯЧПЕФЪЧ______ЃЌгУРызгЗНГЬЪНжЄУї______ЁЃ
ЁОД№АИЁП![]() ЕкЖўжмЦкЂѕAзх
ЕкЖўжмЦкЂѕAзх ![]() Рызг РызгМќЁЂЙВМлМќ
Рызг РызгМќЁЂЙВМлМќ ![]() 2Na2O2+2H2O=4Na++4OH-+O2Ёќ
2Na2O2+2H2O=4Na++4OH-+O2Ёќ ![]() Cl Cl2+H2S=2Cl-+SЁ§+2H+
Cl Cl2+H2S=2Cl-+SЁ§+2H+
ЁОНтЮіЁП
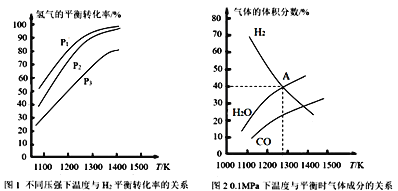
ЖЬжмЦкжїзхдЊЫиAЁЂBЁЂCЁЂDЁЂEдзгађЪ§вРДЮдіДѓЃЛAгыBПЩаЮГЩ4КЫ10ЕчзгЕФЗжзгЃЌдђAЮЊHЃЌBЮЊNдЊЫиЃЛCЁЂEЭЌжїзхЃЌDЁЂEЭЌжмЦкЃЛCКЭDПЩаЮГЩРызгЛЏКЯЮя![]() ЃЌ
ЃЌ![]() жавѕбєРызгЕФЕчзгВуНсЙЙЯрЭЌЃЌЧвЕчзгзмЪ§ЮЊ30ЃЌдђCЮЊOЁЂDЮЊNaдЊЫиЃЛCЁЂEЭЌжїзхЃЌдђEЮЊSдЊЫиЃЌFЕФдзгађЪ§ДѓгкSЃЌдђFЮЊClдЊЫиЃЌОнДЫНтД№ЁЃ
жавѕбєРызгЕФЕчзгВуНсЙЙЯрЭЌЃЌЧвЕчзгзмЪ§ЮЊ30ЃЌдђCЮЊOЁЂDЮЊNaдЊЫиЃЛCЁЂEЭЌжїзхЃЌдђEЮЊSдЊЫиЃЌFЕФдзгађЪ§ДѓгкSЃЌдђFЮЊClдЊЫиЃЌОнДЫНтД№ЁЃ
ИљОнЗжЮіПЩжЊЃКAЮЊHЃЌBЮЊNЃЌCЮЊOЃЌDЮЊNaЃЌEЮЊSдЊЫиЃЌFЮЊClдЊЫиЃЛ
ЃЈ1ЃЉдЊЫиAЕФвЛжжЭЌЮЛЫижЪзгЪ§гыжазгЪ§ЯрЕШЃЌЦфжЪСПЪ§ЮЊ2ЃЌетжжЭЌЮЛЫиЕФЗћКХЪЧ![]() ЃЛ
ЃЛ
ЃЈ2ЃЉNЕФдзгађЪ§ЮЊ7ЃЌЮЛгкжмЦкБэжаЕкЖўжмЦкЂѕAзхЃЛ
ЃЈ3ЃЉдЊЫиHгыNаЮГЩ10ЕчзгЕФЗжзгЮЊ![]() ЃЌЦфЫЎШмвКГЪМюадЃЌгУЕчРыЗНГЬЪНБэЪОЮЊЃК
ЃЌЦфЫЎШмвКГЪМюадЃЌгУЕчРыЗНГЬЪНБэЪОЮЊЃК![]() ЃЛ
ЃЛ
ЃЈ4ЃЉHЁЂNЁЂOаЮГЩЕФдзгИіЪ§4ЃК2ЃК3ЕФбЮЕФЛЏбЇЪНЮЊ![]() ЃЌЪєгкРызгЛЏКЯЮяЃЌКЌгаРызгМќКЭЙВМлМќЃЛ
ЃЌЪєгкРызгЛЏКЯЮяЃЌКЌгаРызгМќКЭЙВМлМќЃЛ
ЃЈ5ЃЉдЊЫиOЁЂNaаЮГЩЛЏКЯЮяЮЊ![]() ЃЌЪєгкРызгЛЏКЯЮяЃЌЦфЕчзгЪНЮЊ
ЃЌЪєгкРызгЛЏКЯЮяЃЌЦфЕчзгЪНЮЊ![]() ЃЌЙ§бѕЛЏФЦгыЫЎЗДгІЩњГЩЧтбѕЛЏФЦКЭбѕЦјЃЌИУЗДгІЕФРызгЗНГЬЪНЮЊЃК2Na2O2+2H2O=4Na++4OH-+O2ЁќЃЛ
ЃЌЙ§бѕЛЏФЦгыЫЎЗДгІЩњГЩЧтбѕЛЏФЦКЭбѕЦјЃЌИУЗДгІЕФРызгЗНГЬЪНЮЊЃК2Na2O2+2H2O=4Na++4OH-+O2ЁќЃЛ
ЃЈ6ЃЉЕчзгВудНЖрЃЌРызгАыОЖдНДѓЃЌОпгаЯрЭЌЕчзгХХВМЕФРызгжадзгађЪ§ДѓЕФРызгАыОЖаЁЃЌдђOЁЂNaЁЂSЁЂClЫљаЮГЩЕФМђЕЅРызгЕФАыОЖгЩаЁЕНДѓЕФЫГађЮЊЃК![]() ЃЛ
ЃЛ
ЃЈ7ЃЉЭЌвЛжмЦкДгзѓЯђгвЗЧН№Ъєадж№НЅдіЧПЃЌЕУЕчзгФмСІж№НЅдіЧПЃЌдђдЊЫидзгЕУЕчзгФмСІНЯЧПЕФЪЧClЃЌИљОнЗДгІCl2+H2S=2Cl-+SЁ§+2H+ПЩжЊЃЌТШЦјбѕЛЏадДѓгкSЃЌдђЗЧН№ЪєадЃК![]() ЁЃ
ЁЃ

 дФЖСПьГЕЯЕСаД№АИ
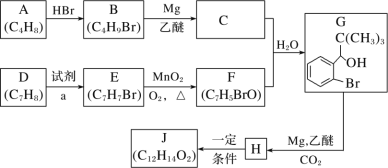
дФЖСПьГЕЯЕСаД№АИЁОЬтФПЁП(1)0.3 molЕФЦјЬЌИпФмШМСЯввХ№Эщ(B2H6)дкбѕЦјжаШМЩеЃЌЩњГЩЙЬЬЌЕФШ§бѕЛЏЖўХ№КЭвКЬЌЫЎЃЌЗХГі649.5 kJШШСПЃЌЦфШШЛЏбЇЗНГЬЪНЮЊ____ЁЃ
(2)ГЌвєЫйЗЩЛњдкЦНСїВуЗЩааЪБЃЌЮВЦјжаЕФNOЛсЦЦЛЕГєбѕВуЁЃПЦбЇМве§дкбаОПРћгУДпЛЏММЪѕНЋЮВЦјжаЕФNOКЭCOзЊБфГЩCO2КЭN2ЃЌЛЏбЇЗНГЬЪНЮЊ2NOЃЋ2CO![]() 2CO2ЃЋN2ЃЌЮЊСЫВтЖЈдкФГжжДпЛЏМСзїгУЯТЕФЗДгІЫйТЪЃЌдкФГЮТЖШЯТгУЦјЬхДЋИаЦїВтЕУВЛЭЌЪБМфЕФNOКЭCOХЈЖШШчБэЃК
2CO2ЃЋN2ЃЌЮЊСЫВтЖЈдкФГжжДпЛЏМСзїгУЯТЕФЗДгІЫйТЪЃЌдкФГЮТЖШЯТгУЦјЬхДЋИаЦїВтЕУВЛЭЌЪБМфЕФNOКЭCOХЈЖШШчБэЃК
ЪБМф/s | 0 | 1 | 2 |
c(NO)/ molЁЄLЃ1 | 1.00ЁС10Ѓ3 | 4.50ЁС10Ѓ4 | 2.50ЁС10Ѓ4 |
c(CO)/molЁЄLЃ1 | 3.60ЁС10Ѓ3 | 3.05ЁС10Ѓ3 | 2.85ЁС10Ѓ3 |
ЪБМф/s | 3 | 4 | 5 |
c(NO)/ molЁЄLЃ1 | 1.50ЁС10Ѓ4 | 1.00ЁС10Ѓ4 | 1.00ЁС10Ѓ4 |
c(CO)/ molЁЄLЃ1 | 2.75ЁС10Ѓ3 | 2.70ЁС10Ѓ3 | 2.70ЁС10Ѓ3 |
ЧыЛиД№ЯТСаЮЪЬтЃК
ЂйЧА2sФкЕФЦНОљЗДгІЫйТЪІд(N2)ЃН____ЃЛ
ЂкЩЯЪіЬѕМўЯТЃЌИУЗДгІЕФЦНКтГЃЪ§ЮЊ____ЃЛ
ЂлЩЯЪіЬѕМўЯТЃЌВтЕУФГЪБПЬЗДгІЬхЯЕжаИїЮяжЪЕФЮяжЪЕФСПХЈЖШОљЮЊ0.01 mol/LЃЌдђДЫЪБЗДгІДІгк____зДЬЌЁЃ(ЬюЁАЦНКтЁБЛђЁАЯђгвНјааЁБЛђЁАЯђзѓНјааЁБ)
(3)ЪЕбщЪвГЃгУ0.10 mol/L KMnO4БъзМЫсадШмвКРДВтЖЈH2C2O4бљЦЗЕФДПЖШ(БъзМвКЕЮД§ВтвК)ЃЌЦфЗДгІдРэЮЊЃК5H2C2O4ЃЋ2MnO4ЃЃЋ6HЃЋ=10CO2ЁќЃЋ2Mn2+ЃЋ8H2OЁЃ
ЂйKMnO4БъзМвКгІзАдк____(ЬюЁАЫсЪНЁБЛђЁАМюЪНЁБ)ЕЮЖЈЙмЃЛ
Ђк ЧхЫЎЯДОЛЕЮЖЈЙмКѓжБНгзАШыБъзМвКЃЌдђВтЖЈНсЙћЛс____ЃЛ(ЬюЁАЦЋДѓЁБЛђЁАЦЋаЁЁБЛђЁАВЛБфЁБ)
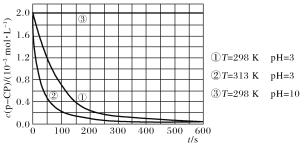
Ђл ЕЮЖЈЙ§ГЬжаЗЂЯжвЛЖЮЪБМфКѓЗДгІЫйТЪУїЯдМгПьЃЌГ§ШЅЮТЖШЕФгАЯьЃЌФуШЯЮЊзюгаПЩФмЕФдвђЪЧ____ЁЃ
ЁОЬтФПЁПБъКХЮЊЂй-ЂтЕФдЊЫиЃЌдкдЊЫижмЦкБэжаЕФЮЛжУШчЯТЃК
жїзх жмЦк | ЂёA | ЂђA | ЂѓA | ЂєA | ЂѕA | ЂіA | ЂїA | 0зх |
1 | Ђй | Ђк | ||||||
2 | Ђл | Ђм | Ђн | Ђо | ||||
3 | Ђп | Ђр | Ђс | Ђт |
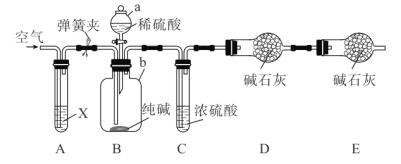
ЪдЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЂкКХдЊЫиЪЧ______![]() ЬюдЊЫиЗћКХ
ЬюдЊЫиЗћКХ![]() ЃЌЂсКХдЊЫиЕФРызгНсЙЙЪОвтЭМЮЊ______
ЃЌЂсКХдЊЫиЕФРызгНсЙЙЪОвтЭМЮЊ______
![]() ШЮаДСНжж
ШЮаДСНжж![]() ЁЃ
ЁЃ
ЃЈ3ЃЉгУЕчзгЪНБэЪОЂйЂмКХдЊЫиаЮГЩЕФзюМђЕЅЛЏКЯЮяЕФаЮГЩЙ§ГЬ______
ЃЈ4ЃЉЂлЕФзюИпМлбѕЛЏЮягыЂрЕФЕЅжЪдкЕуШМЬѕМўЯТЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪН______
ЃЈ5ЃЉЂйЁЂЂнЁЂЂпКХдЊЫиаЮГЩЕФЛЏКЯЮяЕФЕчзгЪНЪЧ______ЃЌИУЛЏКЯЮяЫљКЌЛЏбЇМќЕФРраЭЮЊ______