��Ŀ����
ç�����Ǻϳ����������е�ҩ��--��ƣ�Tamiflu����ԭ��֮һ��ç������A��һ��ͬ���칹�壮A�Ľṹ��ʽ���£�

��1��A�ķ���ʽ�� ��
��2��A��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽ�� ���л��������ýṹ��ʽ��ʾ����
��3��A������������Һ��Ӧ�Ļ�ѧ����ʽ�� ���л��������ýṹ��ʽ��ʾ����
��4��A��Ũ���������¼��ȿɵõ�B��B�Ľṹ��ʽΪ �����䷴Ӧ������ ��
�����䷴Ӧ������ ��
��5��B��ͬ���칹���мȺ��з��ǻ��ֺ��������Ĺ��� �֣�д������һ��ͬ���칹��Ľṹ��ʽ ��
��6��17.4g A������̼��������Һ��Ӧ�������ɶ�����̼�����Ϊ ����״������

��1��A�ķ���ʽ��
��2��A��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽ��
��3��A������������Һ��Ӧ�Ļ�ѧ����ʽ��
��4��A��Ũ���������¼��ȿɵõ�B��B�Ľṹ��ʽΪ
 �����䷴Ӧ������
�����䷴Ӧ��������5��B��ͬ���칹���мȺ��з��ǻ��ֺ��������Ĺ���
��6��17.4g A������̼��������Һ��Ӧ�������ɶ�����̼�����Ϊ
���㣺�л���Ľṹ������
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
��������1������̼ԭ���γ�4����ѧ�����ṹ��ʽ��������ʽ��
��2��A�к�C=C��������ˮ�����ӳɷ�Ӧ��
��3��A�к����Ȼ��������������Ʒ����кͷ�Ӧ��
��4��A�к�-OH�����ô���������������
��5��B��ͬ���칹���мȺ��з��ǻ��ֺ�����������ȡ����Ϊ-OOCH��-OH�������ڡ��䡢��λ�ã�
��6�������A�����ʵ������ɼ�����������������
��2��A�к�C=C��������ˮ�����ӳɷ�Ӧ��
��3��A�к����Ȼ��������������Ʒ����кͷ�Ӧ��
��4��A�к�-OH�����ô���������������
��5��B��ͬ���칹���мȺ��з��ǻ��ֺ�����������ȡ����Ϊ-OOCH��-OH�������ڡ��䡢��λ�ã�
��6�������A�����ʵ������ɼ�����������������
���
�⣺��1����̼ԭ���γ�4����ѧ�����ṹ��ʽ��֪�����л���ķ���ʽΪC7H10O5���ʴ�Ϊ��C7H10O5��
��2��A��������Ȼ�̼��Һ�����ӳɷ�Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��3��A�к�-COOH����NaOH�����кͷ�ӦΪ ��
��
�ʴ�Ϊ�� ��
��
��4��A��Ũ���������¼��ȿɵõ�B��Ϊ����Ũ���������·�������ȥ��Ӧ���ʴ�Ϊ����ȥ��Ӧ��
��5��B�ķ���ʽΪC7H6O3������ֻ�ܺ���1��-COO-����������1��-OH�����ǻ��������ߵ����λ�����ڡ��䡢�������������Ӧ���ʵķֱ�Ϊ ��
��
�ʴ�Ϊ��3�� ��
��
��6��A��NaHCO3�����ķ�Ӧ���Ա�ʾΪ��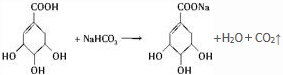 �����ɶ�����̼�����Ϊ
�����ɶ�����̼�����Ϊ
��22.4L/mol=2.24L��
�ʴ�Ϊ��2.24L��
��2��A��������Ȼ�̼��Һ�����ӳɷ�Ӧ�Ļ�ѧ����ʽΪ
 ��
���ʴ�Ϊ��
 ��
����3��A�к�-COOH����NaOH�����кͷ�ӦΪ
 ��
���ʴ�Ϊ��
 ��
����4��A��Ũ���������¼��ȿɵõ�B��Ϊ����Ũ���������·�������ȥ��Ӧ���ʴ�Ϊ����ȥ��Ӧ��
��5��B�ķ���ʽΪC7H6O3������ֻ�ܺ���1��-COO-����������1��-OH�����ǻ��������ߵ����λ�����ڡ��䡢�������������Ӧ���ʵķֱ�Ϊ
 ��
���ʴ�Ϊ��3��
 ��
����6��A��NaHCO3�����ķ�Ӧ���Ա�ʾΪ��
 �����ɶ�����̼�����Ϊ
�����ɶ�����̼�����Ϊ| 17.4g |
| 174g/mol |
�ʴ�Ϊ��2.24L��
�����������ۺϿ����л���Ľṹ�����ʣ��Ǹ߿��еij������ͣ������ۺ���ǿ���ѶȽϴ�ѧ����˼ά��������˸��ߵ�Ҫ������ע�ػ���֪ʶ������ѵ����ͬʱ�������������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�����ͳ���˼ά���������ѧ���ķ������⡢��������������

��ϰ��ϵ�д�
�����Ŀ
�����½�ϡNaOH��Һ��ϡCH3COOH��Һ��ϣ������ܳ��ֵĽ���ǣ�������
| A��PH��7��c��Na+����c��CH3COO-����c��OH-����c��H+�� |
| B��PH��7��c��Na+��+c��H+���Tc��OH-��+c��CH3COO-�� |
| C��PH��7��c��CH3COO-����c��H+����c��Na+����c��OH-�� |
| D��PH=7��c��CH3COO-����c��Na+����c��H+���Tc��OH-�� |
���и�����̬�⻯���У����ȶ��Ե�����˳�����е�һ���ǣ�������
| A��SiH4��PH3��HCl��HF |
| B��NH3��HF��PH3��HCl |
| C��SiH4��PH3��H2O��H2S |
| D��CH4��NH3��PH3��HCl |
����˵����ȷ���ǣ�������
| A����������ͬ�����ֲ�ͬ����һ����Ϊͬ���칹�� |
| B����Ԫ��������������ͬ�����ֲ�ͬ����һ����Ϊͬ���칹�� |
| C��ͬ���칹�����������һ����ͬ |
| D����Ϊͬϵ������ַ��Ӳ����ܻ�Ϊͬ���칹�� |