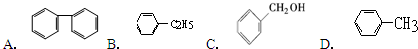��Ŀ����
��1�����и����е������л����������ͬ�����ʡ�ͬϵ���ͬ���칹��ȣ����ж�����֮��Ĺ�ϵ
��2-������Ͷ��� ��
��1-��ϩ�ͻ����� ��
��2��֧��ֻ��һ���һ���ʽ����С�������Ľṹ��ʽ ��
��3��д����ȩ��Һ��������������Һ���ȵĻ�ѧ����ʽ�� ��
��4��д��1��3-����ϩ���嵥�ʷ���1��4-�ӳɵķ�Ӧ����ʽ
��5��д�����Ҵ�һ����������Ļ�ѧ����ʽ ��
��2-������Ͷ���
��1-��ϩ�ͻ�����
��2��֧��ֻ��һ���һ���ʽ����С�������Ľṹ��ʽ
��3��д����ȩ��Һ��������������Һ���ȵĻ�ѧ����ʽ��
��4��д��1��3-����ϩ���嵥�ʷ���1��4-�ӳɵķ�Ӧ����ʽ
��5��д�����Ҵ�һ����������Ļ�ѧ����ʽ
���㣺������������,��ѧ����ʽ����д
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�,�л���������ͨʽ��Ӧ�ù���
��������1���ṹ���ƣ��ڷ�����������һ�������ɸ�CH2ԭ���ŵ����ʻ���ͬϵ�����ʽ��ͬ�ṹ��ͬ�Ļ����ﻥ��ͬ���칹�壬���ݶ�������жϣ�
��2�������к���ȡ�����һ������������ٺ���5��Cԭ�ӣ��ݴ�д�����������������Ľṹ��ʽ��
��3����ȩ��������Һ��Ӧ�����������ʡ�����李�������ˮ��
��4��1��3-����ϩ���嵥�ʷ���1��4-�ӳ�����CH2BrCH=CHCH2Br��
��5���Ҵ����廯���ڼ��������·���ȡ����Ӧ�����������ˮ��
��2�������к���ȡ�����һ������������ٺ���5��Cԭ�ӣ��ݴ�д�����������������Ľṹ��ʽ��
��3����ȩ��������Һ��Ӧ�����������ʡ�����李�������ˮ��
��4��1��3-����ϩ���嵥�ʷ���1��4-�ӳ�����CH2BrCH=CHCH2Br��
��5���Ҵ����廯���ڼ��������·���ȡ����Ӧ�����������ˮ��
���
�⣺��1����2-������Ͷ��鶼�����������ҽṹ���ƣ��ڷ�����������һ��CH2ԭ���ţ�����2-������Ͷ�������ͬϵ�
�ʴ�Ϊ��ͬϵ�
��1-��ϩ�ͻ�����ķ���ʽΪC6H12�������ʽ��ͬ���ṹ��ͬ����������ͬ���칹�壬
�ʴ�Ϊ��ͬ���칹�壻
CH2CH3
��2���һ�������3��λ��ֻ��һ���һ������������ٺ���5��C����ʽ����С�������Ľṹ��ʽΪ��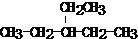 ��
��
�ʴ�Ϊ��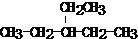 ��
��
��3����ȩ��������Һ��Ӧ���ɴ���李������ʡ�������ˮ����Ӧ�����ӷ���ʽΪ��CH3CHO+2Ag��NH3��2OH
CH3COONH4+H2O+2Ag��+3NH3��
�ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH
CH3COONH4+H2O+2Ag��+3NH3��
��4��1��3-����ϩ���嵥�ʷ���1��4-�ӳɷ�Ӧ�ķ�Ӧ����ʽΪ��CH2=CH-CH=CH2+Br2��CH2BrCH=CHCH2Br��
�ʴ�Ϊ��CH2=CH-CH=CH2+Br2��CH2BrCH=CHCH2Br��
��5�����Ҵ�һ���������飬�������Ҵ����廯�ⷢ��ȡ����Ӧ��ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH+HBr
CH3CH2Br+H2O��
�ʴ�Ϊ��CH3CH2OH+HBr
CH3CH2Br+H2O��
�ʴ�Ϊ��ͬϵ�
��1-��ϩ�ͻ�����ķ���ʽΪC6H12�������ʽ��ͬ���ṹ��ͬ����������ͬ���칹�壬
�ʴ�Ϊ��ͬ���칹�壻
CH2CH3
��2���һ�������3��λ��ֻ��һ���һ������������ٺ���5��C����ʽ����С�������Ľṹ��ʽΪ��
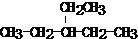 ��
���ʴ�Ϊ��
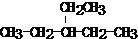 ��
����3����ȩ��������Һ��Ӧ���ɴ���李������ʡ�������ˮ����Ӧ�����ӷ���ʽΪ��CH3CHO+2Ag��NH3��2OH
| �� |
�ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH
| �� |
��4��1��3-����ϩ���嵥�ʷ���1��4-�ӳɷ�Ӧ�ķ�Ӧ����ʽΪ��CH2=CH-CH=CH2+Br2��CH2BrCH=CHCH2Br��
�ʴ�Ϊ��CH2=CH-CH=CH2+Br2��CH2BrCH=CHCH2Br��
��5�����Ҵ�һ���������飬�������Ҵ����廯�ⷢ��ȡ����Ӧ��ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH+HBr
| �� |
�ʴ�Ϊ��CH3CH2OH+HBr
| �� |
���������⿼����ͬϵ����ͬ���칹����жϡ������л���Ӧ����ʽ����д����Ŀ�Ѷ��еȣ�ע�����ճ����л���ṹ�����ʣ���ȷ�����л���Ӧ���ͣ��ܹ���ȷ��д�����л���Ӧ�ķ���ʽ��

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ
�����£���pH=8��NaOH��pH=10��NaOH��Һ�������Ϻ���Һ��c��H+����ӽ��ڣ�������
| A����10-8+10-10��mol/L | ||
| B����10-4+10-6��mol/L | ||
C��
| ||
| D��2��10-10mol/L |
ʵ��������й��ˡ��������������ʵ���Ũ�ȵIJ���ʱ����Ҫ�õ��������ǣ�������
| A���ձ� | B�������� |
| C�������� | D���ƾ��� |
���з�Ӧ�У�ˮ�Ȳ����������ֲ�����ԭ����������ԭ��Ӧ�ǣ�������
A��C+H2O
| ||||
B��2H2O
| ||||
| C��Na2O+H2O�T2NaOH | ||||
| D��3NO2+H2O�T2HNO3+NO |
 ijУ��ѧ�о���ѧϰС��ѧϰ�˻�ѧ��Ӧ�����������ݺ�CaCO3��ϡ����ķ�Ӧ���������̽�������������������£���CaCO3��״�������1L��1mol?L-1ϡ�����У���¼�����淴Ӧʱ��ı仯����CO2��������ʵ�����������Ƴ���ͼ���ߣ�������Һ������仯���Բ��ƣ�������������⣺
ijУ��ѧ�о���ѧϰС��ѧϰ�˻�ѧ��Ӧ�����������ݺ�CaCO3��ϡ����ķ�Ӧ���������̽�������������������£���CaCO3��״�������1L��1mol?L-1ϡ�����У���¼�����淴Ӧʱ��ı仯����CO2��������ʵ�����������Ƴ���ͼ���ߣ�������Һ������仯���Բ��ƣ�������������⣺
 �����䷴Ӧ������
�����䷴Ӧ������