��Ŀ����
��֪��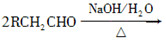
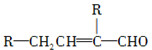
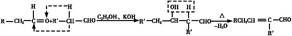
ˮ����EΪ���������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
��ش��������⣺
(1)һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ________���ṹ������ʾAֻ��һ������A������Ϊ________��
(2)B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ__________________________��
(3)C��________�ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ���________��
(4)�ڢ۲��ķ�Ӧ����Ϊ________��D���������ŵ�����Ϊ________��
(5)д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ��__________________________��
a����������6��̼ԭ����һ��ֱ���ϣ�
b�����������������Ű���ˮ������еĹ����š�
(6)�ڢܲ��ķ�Ӧ����Ϊ________��д��E�Ľṹ��ʽ��______________��
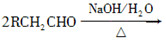
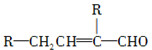
ˮ����EΪ���������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�

��ش��������⣺
(1)һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ________���ṹ������ʾAֻ��һ������A������Ϊ________��
(2)B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ__________________________��
(3)C��________�ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ���________��
(4)�ڢ۲��ķ�Ӧ����Ϊ________��D���������ŵ�����Ϊ________��
(5)д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ��__________________________��
a����������6��̼ԭ����һ��ֱ���ϣ�
b�����������������Ű���ˮ������еĹ����š�
(6)�ڢܲ��ķ�Ӧ����Ϊ________��д��E�Ľṹ��ʽ��______________��
(1)C4H10O��1������(��������)
(2)CH3CH2CH2CHO��2Cu(OH)2��NaOH CH3CH2CH2COONa��Cu2O����3H2O
CH3CH2CH2COONa��Cu2O����3H2O
(3)2��������Һ��ϡ���ᡢ��ˮ(������������)
(4)��ԭ��Ӧ(��ӳɷ�Ӧ)���ǻ�

(6)ŨH2SO4������

(2)CH3CH2CH2CHO��2Cu(OH)2��NaOH
 CH3CH2CH2COONa��Cu2O����3H2O
CH3CH2CH2COONa��Cu2O����3H2O(3)2��������Һ��ϡ���ᡢ��ˮ(������������)
(4)��ԭ��Ӧ(��ӳɷ�Ӧ)���ǻ�

(6)ŨH2SO4������

�����ṩ��Ϣ��ת������ȷ����Ӧ�P��Ӧ���͡�
(1)AΪһԪ��������������������ԼΪ21.6%��������Է�������Mr��16��21.6%��74�������ʽΪCxHyO����12x��y��16��74,12x��y��58����x��4ʱ��y��10������ʽΪC4H10O����x��3��x��5ʱ��������������A�ķ���ʽΪC4H10O��A��ֻ��һ����������AΪCH3CH2CH2CH2OH������Ϊ1������(��������)��(2)�ɢ�������֪BΪCH3CH2CH2CHO��B�����Ƶ�Cu(OH)2�ķ�ӦΪCH3CH2CH2CHO��2Cu(OH)2��NaOH CH3CH2CH2COONa��Cu2O����3H2O��
CH3CH2CH2COONa��Cu2O����3H2O��
(3)������ṩ����Ϣ��֪�ڵķ�Ӧ����Ϊ

����C��2�ֽṹ����CHO�� ��ͬ����ʱҪ�ȼ��飭CHO��Ȼ�����
��ͬ����ʱҪ�ȼ��飭CHO��Ȼ����� �������Լ�Ϊ������Һ��ϡ�������ˮ��(4)C����Է�������Ϊ126��D����Է�������Ϊ130������C��D����H2�����ӳɷ�Ӧ��Ҳ��Ϊ��ԭ��Ӧ��������Է����������4������
�������Լ�Ϊ������Һ��ϡ�������ˮ��(4)C����Է�������Ϊ126��D����Է�������Ϊ130������C��D����H2�����ӳɷ�Ӧ��Ҳ��Ϊ��ԭ��Ӧ��������Է����������4������ �ͣ�CHO����H2�ӳɣ�����D�й���������Ϊ�ǻ���
�ͣ�CHO����H2�ӳɣ�����D�й���������Ϊ�ǻ���

(6)�ڢܲ�Ϊ������Ӧ������������ŨH2SO4�����ȣ�E�Ľṹ��ʽΪ ��
��
�㲦��֪ʶ������ʽ��ȷ�����л���Ӧ���͡�ͬ���칹�塢�л���ļ��顢��ѧ����ʽ����д��D����������ѧ���������л�ȡ��Ϣ���������ۺϷ����жϽ�����⡢��ȷ������ۺ������������Ѷȣ��ϴ�
(1)AΪһԪ��������������������ԼΪ21.6%��������Է�������Mr��16��21.6%��74�������ʽΪCxHyO����12x��y��16��74,12x��y��58����x��4ʱ��y��10������ʽΪC4H10O����x��3��x��5ʱ��������������A�ķ���ʽΪC4H10O��A��ֻ��һ����������AΪCH3CH2CH2CH2OH������Ϊ1������(��������)��(2)�ɢ�������֪BΪCH3CH2CH2CHO��B�����Ƶ�Cu(OH)2�ķ�ӦΪCH3CH2CH2CHO��2Cu(OH)2��NaOH
 CH3CH2CH2COONa��Cu2O����3H2O��
CH3CH2CH2COONa��Cu2O����3H2O��(3)������ṩ����Ϣ��֪�ڵķ�Ӧ����Ϊ

����C��2�ֽṹ����CHO��
 ��ͬ����ʱҪ�ȼ��飭CHO��Ȼ�����
��ͬ����ʱҪ�ȼ��飭CHO��Ȼ����� �������Լ�Ϊ������Һ��ϡ�������ˮ��(4)C����Է�������Ϊ126��D����Է�������Ϊ130������C��D����H2�����ӳɷ�Ӧ��Ҳ��Ϊ��ԭ��Ӧ��������Է����������4������
�������Լ�Ϊ������Һ��ϡ�������ˮ��(4)C����Է�������Ϊ126��D����Է�������Ϊ130������C��D����H2�����ӳɷ�Ӧ��Ҳ��Ϊ��ԭ��Ӧ��������Է����������4������ �ͣ�CHO����H2�ӳɣ�����D�й���������Ϊ�ǻ���
�ͣ�CHO����H2�ӳɣ�����D�й���������Ϊ�ǻ���
(6)�ڢܲ�Ϊ������Ӧ������������ŨH2SO4�����ȣ�E�Ľṹ��ʽΪ
 ��
���㲦��֪ʶ������ʽ��ȷ�����л���Ӧ���͡�ͬ���칹�塢�л���ļ��顢��ѧ����ʽ����д��D����������ѧ���������л�ȡ��Ϣ���������ۺϷ����жϽ�����⡢��ȷ������ۺ������������Ѷȣ��ϴ�

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ
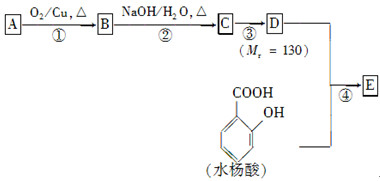
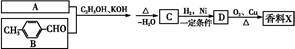
 ����һ��ҽҩ�ϳ��м��壬ijͬѧ������ĺϳ�·�����£�
����һ��ҽҩ�ϳ��м��壬ijͬѧ������ĺϳ�·�����£�
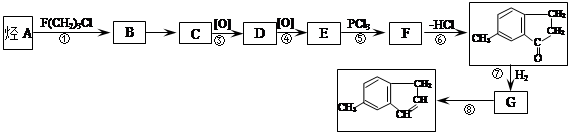

 �ĸ߾���䵥��Ӧ�ǣ��ٱ���ϩ���ڱ�ϩ����2��ϩ����1��ϩ������ϩ���ޱ���ϩ( )
�ĸ߾���䵥��Ӧ�ǣ��ٱ���ϩ���ڱ�ϩ����2��ϩ����1��ϩ������ϩ���ޱ���ϩ( )

 )��ʳƷ���Ӽ�������ԭ�ϡ�
)��ʳƷ���Ӽ�������ԭ�ϡ�
 RCHO��
RCHO�� )��һ��ҽҩ�м��塣������ȩ(
)��һ��ҽҩ�м��塣������ȩ( )�ϳ�E(����ԭ����ѡ)���漰�ķ�Ӧ������(����Ӧ˳����д)________________________��
)�ϳ�E(����ԭ����ѡ)���漰�ķ�Ӧ������(����Ӧ˳����д)________________________��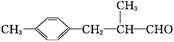

 ���������
���������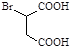 ��
��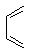 Ϊԭ�Ϻϳɻ�����
Ϊԭ�Ϻϳɻ�����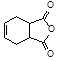 ��ʵ�鷽�������úϳ�·������ͼ��ʾΪ��A
��ʵ�鷽�������úϳ�·������ͼ��ʾΪ��A B����
B����
