��Ŀ����
(6��)KMnO4��Һ��H2C2O4��Һ�ɷ������·�Ӧ��
2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O
��1���÷�Ӧ���ʿ�ʼʮ�ֻ�����һ��ʱ���ͻȻ�ӿ죬������Ϊ_____(�ѧʽ)�Ը÷�Ӧ���д����á�
��2���ݴ�ԭ������������KMnO4��Һ���ⶨH2C2O4��Һ��Ũ�ȣ������������£�
��ȷ����0.10mol/L��KMnO4��Һ
�ڽ�KMnO4��Һʢ����______�ζ����У����ʽ����ʽ����
��ȷ��ȡ25.00mL H2C2O4��Һ����ƿ��
�ܽ��еζ�
�ζ��յ���ʲô����_________________,�Ƿ���Ҫָʾ��__________(��ǡ���)
��3�������в����У���ʹ�ⶨ��H2C2O4��ҺŨ��ƫ�����___________��
��ʢװKMnO4��Һ�ĵζ���������ˮϴ����δ��KMnO4��Һ��ϴ
����ƿ��ʢ����������ˮ���ټӴ���Һ
��ʢװH2C2O4��Һ�ĵζ���������ˮϴ����δ��H2C2O4��Һ��ϴ
�ܵζ���۲�ζ��ܶ���ʱ�����߸��ڿ̶���
��4���ζ�ʱ���õ�ʵ���������£��Լ�������H2C2O4��Һ��Ũ��Ϊ_________mol/L
2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O
��1���÷�Ӧ���ʿ�ʼʮ�ֻ�����һ��ʱ���ͻȻ�ӿ죬������Ϊ_____(�ѧʽ)�Ը÷�Ӧ���д����á�
��2���ݴ�ԭ������������KMnO4��Һ���ⶨH2C2O4��Һ��Ũ�ȣ������������£�
��ȷ����0.10mol/L��KMnO4��Һ
�ڽ�KMnO4��Һʢ����______�ζ����У����ʽ����ʽ����
��ȷ��ȡ25.00mL H2C2O4��Һ����ƿ��
�ܽ��еζ�
�ζ��յ���ʲô����_________________,�Ƿ���Ҫָʾ��__________(��ǡ���)
��3�������в����У���ʹ�ⶨ��H2C2O4��ҺŨ��ƫ�����___________��
��ʢװKMnO4��Һ�ĵζ���������ˮϴ����δ��KMnO4��Һ��ϴ
����ƿ��ʢ����������ˮ���ټӴ���Һ
��ʢװH2C2O4��Һ�ĵζ���������ˮϴ����δ��H2C2O4��Һ��ϴ
�ܵζ���۲�ζ��ܶ���ʱ�����߸��ڿ̶���
��4���ζ�ʱ���õ�ʵ���������£��Լ�������H2C2O4��Һ��Ũ��Ϊ_________mol/L
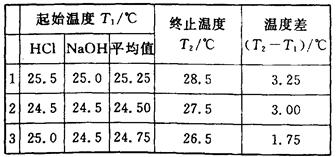
| ʵ�������� | ����Һ���mL | ����ı�Һ�����mL�� |
| 1 | 25.00 | 28.95 |
| 2 | 25.00 | 25.05 |
| 3 | 25.00 | 24.95 |
��6�֣���1��MnSO4 ��2����ʽ ��Һ����ɫ��Ϊ�Ϻ�ɫ �� ��3���� ��4��0.25
��1�����ݷ���ʽ��֪�������ɵ������������̺�CO2����CO2�����壬����������õ��������̡�
��2�����������Һ����ǿ�����ԣ��ܸ�ʴ������Ӧ������ʽ�ζ���ʢ�Ÿ��������Һ���������Ը��������Һ�����Ϻ�ɫ�ģ����Բ���Ҫ����ָʾ���յ�ʱ����������Һ����ɫ��Ϊ�Ϻ�ɫ��
��3�����൱��ϡ���˸��������Һ��Ũ�ȣ��������ĸ��������Һ�����ƫ�ⶨ���ƫ�ߡ���ƿ�����ñ�Һ��ϴ�����Ԣڲ�Ӱ�졣��Ҳ���൱��ϡ�Ͳ����Ũ�ȣ��������ĸ��������Һ�����ƫ�٣��ⶨ���ƫ�͡��ζ���۲�ζ��ܶ���ʱ�����߸��ڿ̶��ߣ�˵������ƫС�����Բⶨ���ƫ�ͣ���ѡ�١�
��4������ʵ�����ݿ�֪����һ��ʵ�����̫�����á�ʵ����ݺ����ε����ݿ�֪�����ĸ��������Һ�������ƽ��ֵ��25.00ml�����Ը��ݷ���ʽ��֪��������Һ��Ũ����0.10mol/L��2.5��0.25mol/L��
��2�����������Һ����ǿ�����ԣ��ܸ�ʴ������Ӧ������ʽ�ζ���ʢ�Ÿ��������Һ���������Ը��������Һ�����Ϻ�ɫ�ģ����Բ���Ҫ����ָʾ���յ�ʱ����������Һ����ɫ��Ϊ�Ϻ�ɫ��
��3�����൱��ϡ���˸��������Һ��Ũ�ȣ��������ĸ��������Һ�����ƫ�ⶨ���ƫ�ߡ���ƿ�����ñ�Һ��ϴ�����Ԣڲ�Ӱ�졣��Ҳ���൱��ϡ�Ͳ����Ũ�ȣ��������ĸ��������Һ�����ƫ�٣��ⶨ���ƫ�͡��ζ���۲�ζ��ܶ���ʱ�����߸��ڿ̶��ߣ�˵������ƫС�����Բⶨ���ƫ�ͣ���ѡ�١�
��4������ʵ�����ݿ�֪����һ��ʵ�����̫�����á�ʵ����ݺ����ε����ݿ�֪�����ĸ��������Һ�������ƽ��ֵ��25.00ml�����Ը��ݷ���ʽ��֪��������Һ��Ũ����0.10mol/L��2.5��0.25mol/L��

��ϰ��ϵ�д�
�����Ŀ
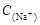 =2[
=2[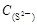 +
+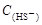 +
+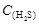 ]
]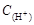 =
=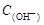 +
+