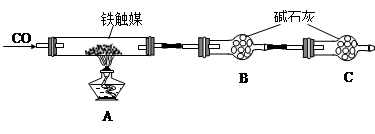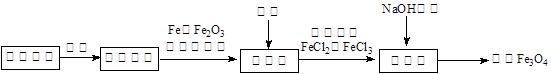��Ŀ����
Ӳ�ʲ������ǻ�ѧʵ���о���ʹ�õ�һ�����������������ʵ�飨�̶�װ���ԣ����ش����⡣
������ʵ�飺��ͼ��ʾ����Ũ�������װ��Na2SO3�����������һ��ʱ���a��b��c��������仯���±�������д���еĿհף�

��Ӳ�ʲ�����������װ�ý����ɶ��Ի���ʵ�顣��ͼ��ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����װ�á�

ʵ��һ������ʯ�к������IJⶨ
a������ͼ��װ����(�г�������ʡ��)�����װ�õ������ԣ�
b����5��0g����ʯ����Ӳ�ʲ������У�
c������˵����ܿڴ����ϵػ���ͨ��H2�� ��ȼA���ƾ��ƣ�
d����ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ����������ȫ��ȴ��
��1������c�����
��2����÷�Ӧ��װ��B����1��35g��������ʯ��������������Ϊ______________________��ʵ���������ʯ�к������IJⶨ

(3)���������е�Ŀ����_____________________________________________��
(4)����ں͢��ж�Ҫ�õ��IJ���������______ __��
(5)�����йز���IJ�����˵������ȷ����_______________________________��
a���ζ���������ˮϴ�Ӻ����ô�װҺ��ϴ
b����ƿ����Ҫ�ô���Һ��ϴ
c����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
ʵ����ۣ�������������������
(6)��ʵ��һ�����ó�������ʯ������������Ļ�ѧʽΪ____________________________��
������ʵ�飺��ͼ��ʾ����Ũ�������װ��Na2SO3�����������һ��ʱ���a��b��c��������仯���±�������д���еĿհף�

| ���� | �����ϵμӵ��Լ� | ʵ������ | ���ͺͽ��� |
| a | | �����ף��Ⱥ��ָֻ���ɫ |  |
| b | ����̪��NaOH��Һ | �����Ϊ��ɫ | ���ӷ���ʽ�� |
| c | | �����Ϊ��ɫ | ���ۣ���������� �� |

ʵ��һ������ʯ�к������IJⶨ
a������ͼ��װ����(�г�������ʡ��)�����װ�õ������ԣ�
b����5��0g����ʯ����Ӳ�ʲ������У�
c������˵����ܿڴ����ϵػ���ͨ��H2�� ��ȼA���ƾ��ƣ�
d����ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ����������ȫ��ȴ��
��1������c�����
��2����÷�Ӧ��װ��B����1��35g��������ʯ��������������Ϊ______________________��ʵ���������ʯ�к������IJⶨ

(3)���������е�Ŀ����_____________________________________________��
(4)����ں͢��ж�Ҫ�õ��IJ���������______ __��
(5)�����йز���IJ�����˵������ȷ����_______________________________��
a���ζ���������ˮϴ�Ӻ����ô�װҺ��ϴ
b����ƿ����Ҫ�ô���Һ��ϴ
c����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
ʵ����ۣ�������������������
(6)��ʵ��һ�����ó�������ʯ������������Ļ�ѧʽΪ____________________________��
��ÿ��1�֣�
��1����Cװ�ó��ڴ��ռ�H2���鴿��1�֣���2��24%��2�֣�
��3����ȥ��Һ���ܽ�Ĺ�����Cl2��1�֣���4���ձ�����������1�֣���5��c��1�֣���6��Fe5O6��2�֣�
| ���� | �����ϵμӵ��Լ� | ʵ������ | ���ͺͽ��� |
| a | Ʒ����Һ | | |
| b | | | ���ӷ���ʽ�� 2OH��+SO2=SO32��+H2O |
| c | �����۵ĵ�ˮ | | ���ۣ���������� ��ԭ �� |
��3����ȥ��Һ���ܽ�Ĺ�����Cl2��1�֣���4���ձ�����������1�֣���5��c��1�֣���6��Fe5O6��2�֣�
�����������a��Ũ�������������Ʒ�Ӧ����SO2��SO2����Ư���ԡ�Ʒ����Һ�Ǻ�ɫ�ģ�����������ʹƷ����ɫ��Ư��ԭ���ǣ�SO2��Ʒ�췴Ӧ������ɫ���ȶ����ʣ����ȷֽ�������SO2��Ʒ�죬�ָֻ���ɫ��b����������������̪Ҳ�Ǻ�ɫ�ģ�SO2������������ܺ��������Ʒ�Ӧ����Һ���Խ��ͣ����º�ɫ��ʧ����Ӧԭ���ǣ�2OH��+SO2=SO32��+H2O��c���ⵥ���������۱�Ϊ��ɫ������������л�ԭ�ԣ��ܽ��ⵥ�ʻ�ԭΪ�����ӣ�SO2+I2+2H2O��H2SO4+2HI���Ӷ�ʹ��ɫ��ʧ��
�������ǿ�ȼ�����壬��ȼǰ��Ӧ�����䴿�ȣ�������Cװ�ó��ڴ��ռ�H2���鴿���ٵ�ȼA���ƾ��ơ�
��2����ʵ���У���������������Ӧ���ɽ�������ˮ�����ݹ��������ı仯���������ĺ�����B���ĸ�������������ղ�����ˮ��������ķ�Ӧ��װ��B����1.35g������Ӧ������ˮ��������1.35g�����ʵ�����1.35g��18g/mol��0.075mol��������Ԫ�ص�������0.075mol��16g/mol��1.2g�����Ը�����ԭ���غ��֪������ʯ����������������
 ��100%��24%��
��100%��24%����3����������ǿ�����ԣ������������ӣ��Ӷ���������������ӵķ�Ӧ��������е������Ǹ�����Һ���ܽ�Ĺ�����Cl2��
��4�����ǹ��ˣ���Ҫ�ձ���������������̨���������ʵ���Ũ�ȵ����ƣ���Ҫ��������250ml����ƿ���ձ�������������ͷ�ιܣ����Բ���ں͢��ж�Ҫ�õ��IJ����������ձ��Ͳ�������
��5��a���ζ���������ˮϴ�Ӻ�����ñ�Һ��ϴ�����a��ȷ��b����ƿ����Ҫ�ô���Һ��ϴ��b��ȷ��c����Ϊ��ˮΪ��ɫ������������Ҳ�ǻ�ɫ��Һ���ζ����������ָʾ����c����ȷ����ѡc����6��������ȡ��Һ����Fe3+�������������KI��Һ�������ȣ���Ϸ���ʽ2Fe3����2I����I2��2Fe2����֪��c��Fe3+����c��KI����0.5mol?L-1��������Ԫ�ص����ʵ�����0.5mol/L��0.25L��0.125mol���������������������ĸ���֮�ȣ�0.125mol:0.075mol��2��6:5����˻�ѧʽΪFe5O6��2�����ʡ�����ʯ��Ԫ�غ����ⶨ��ʵ�������̽���Լ���ѧʽȷ�����йؼ���

��ϰ��ϵ�д�
�����Ŀ