��Ŀ����
2010���Ϻ������������ǡ����У�����������á����Իش��������⣺
��1��Ϊȷ���������ڼ���ǿ������������ʴﵽ95%���ϣ��������ڼ�Ŀ�������״������У�����Ҫ����ָ����
a������������PM10��b��NO2Ũ�� c��SO2Ũ�� d��CO2Ũ�ȣ�
��2����ɳ�����ն�����Ҫ������
��3��Ϊ2010���������ڼ��ṩ��ѧ��Ч�Ļ����������Ϸ�������������ˮ�������������죬������ˮʱ����K2SO4?Al2��SO4��3?24H2O���ʽ�Ȼ�����������
��4���������й���--������֮�ڡ������ɸֽ��������7000����ɫ�����1200��鲣���Ƚ��ɣ������������õĹ�ҵ�豸��
��1��Ϊȷ���������ڼ���ǿ������������ʴﵽ95%���ϣ��������ڼ�Ŀ�������״������У�����Ҫ����ָ����
d
d
��a������������PM10��b��NO2Ũ�� c��SO2Ũ�� d��CO2Ũ�ȣ�
��2����ɳ�����ն�����Ҫ������
���ﰺ���ȷ���������������ȣ�
���ﰺ���ȷ���������������ȣ�
��Ϊ�˸��ƿ���������������ƴ����ж�������������̳�����Ⱦ����ŷ�������������װβ��������װ�ã�ʹ���е��к�����NO��COת��Ϊ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ2NO+2CO
N2+2CO2
| ||
2NO+2CO
N2+2CO2
��
| ||
��3��Ϊ2010���������ڼ��ṩ��ѧ��Ч�Ļ����������Ϸ�������������ˮ�������������죬������ˮʱ����K2SO4?Al2��SO4��3?24H2O���ʽ�Ȼ�����������
������
������
��ͨ�������������ȵ�������������
������
��ijũ�����Ϊ�������ˮ���ڽ��ر�ˮȡ�ؼҺ�ʹ��Ư�ۻ�Ư��Ƭ����ɱ����������ԭ�����û�ѧ����ʽ��ʾΪCa��ClO��2+H2O+CO2=CaCO3��+2HClO
Ca��ClO��2+H2O+CO2=CaCO3��+2HClO
����4���������й���--������֮�ڡ������ɸֽ��������7000����ɫ�����1200��鲣���Ƚ��ɣ������������õĹ�ҵ�豸��
����Ҥ
����Ҥ
��ʯӢ������ѧ�ȶ���ǿ������ϵ��С����һ�����ֲ�����ʯӢ��������Ҫ�ɷ���SiO2
SiO2
����������1�����������ձ��м����ǿ����������Ͷ�������͵������
��2����ɳ�����ն�����Ҫ�����Ƿ��ﰺ ���ȷ���������������ȣ���һ��������һ����̼�ڴ��������·�Ӧ���ɵ����Ͷ�����̼��
��3����������������������������������������Ͷ���������ǿ������������������Ư���ܺͶ�����̼��ˮ��Ӧ���ɴ����ᣬ��������������
��4�������������õĹ�ҵ�豸�Dz���Ҥ��������¯����ʯӢ��������Ҫ�ɷ���SiO2��
��2����ɳ�����ն�����Ҫ�����Ƿ��ﰺ ���ȷ���������������ȣ���һ��������һ����̼�ڴ��������·�Ӧ���ɵ����Ͷ�����̼��
��3����������������������������������������Ͷ���������ǿ������������������Ư���ܺͶ�����̼��ˮ��Ӧ���ɴ����ᣬ��������������
��4�������������õĹ�ҵ�豸�Dz���Ҥ��������¯����ʯӢ��������Ҫ�ɷ���SiO2��
����⣺��1�����������ձ��м����ǿ����������Ͷ�������͵���������Զ�����̼���ڼ�ⷶΧ֮�ڣ���ѡd��
��2����ɳ�����ն�����Ҫ�����Ƿ��ﰺ ���ȷ���������������ȣ���һ��������һ����̼�ڴ��������·�Ӧ���ɵ����Ͷ�����̼����Ӧ����ʽΪ����2NO+2CO
N2+2CO2��
�ʴ�Ϊ�����ﰺ ���ȷ���������������ȣ���2NO+2CO
N2+2CO2��
��3����������ǿ����������ˮ�����������������壬��������������������ԣ�������ˮ�е����������������ˮ����K2SO4?Al2��SO4��3?24H2O���ʽ�Ȼ����������ǻ������������Ͷ���������ǿ��������ɱ����������������������������ƺͶ�����̼��ˮ��Ӧ����̼��ƺʹ����ᣬ��Ӧ����ʽΪ��Ca��ClO��2+H2O+CO2=CaCO3��+2HClO��
�ʴ�Ϊ������������������Ca��ClO��2+H2O+CO2=CaCO3��+2HClO��
��4�������������õĹ�ҵ�豸�Dz���Ҥ��������¯����ʯӢ��������Ҫ�ɷ���SiO2��
�ʴ�Ϊ������Ҥ��������¯����SiO2��
��2����ɳ�����ն�����Ҫ�����Ƿ��ﰺ ���ȷ���������������ȣ���һ��������һ����̼�ڴ��������·�Ӧ���ɵ����Ͷ�����̼����Ӧ����ʽΪ����2NO+2CO
| ||
�ʴ�Ϊ�����ﰺ ���ȷ���������������ȣ���2NO+2CO
| ||
��3����������ǿ����������ˮ�����������������壬��������������������ԣ�������ˮ�е����������������ˮ����K2SO4?Al2��SO4��3?24H2O���ʽ�Ȼ����������ǻ������������Ͷ���������ǿ��������ɱ����������������������������ƺͶ�����̼��ˮ��Ӧ����̼��ƺʹ����ᣬ��Ӧ����ʽΪ��Ca��ClO��2+H2O+CO2=CaCO3��+2HClO��
�ʴ�Ϊ������������������Ca��ClO��2+H2O+CO2=CaCO3��+2HClO��
��4�������������õĹ�ҵ�豸�Dz���Ҥ��������¯����ʯӢ��������Ҫ�ɷ���SiO2��
�ʴ�Ϊ������Ҥ��������¯����SiO2��
���������⿼���˳������������Ⱦ��������ע�������̼���ڿ��������ձ��ļ�ⷶΧ�У�ע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
 2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������lnGaN���������أ�Ϊ������֪������ͬ����һ���ڵ�Ԫ�أ��黯�صľ����ṹ��ͼ���Իش�
2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������lnGaN���������أ�Ϊ������֪������ͬ����һ���ڵ�Ԫ�أ��黯�صľ����ṹ��ͼ���Իش� 2010���Ϻ������᳡�ݴ������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LED��Ƭ���ʻ�����GaAs���黯�أ���AlGaInP��������������InGaN���������أ�Ϊ������֪������ͬ����һ���ڵ�Ԫ�أ��黯�صľ����ṹ��ͼ���Իش�
2010���Ϻ������᳡�ݴ������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LED��Ƭ���ʻ�����GaAs���黯�أ���AlGaInP��������������InGaN���������أ�Ϊ������֪������ͬ����һ���ڵ�Ԫ�أ��黯�صľ����ṹ��ͼ���Իش�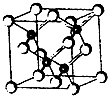 ��2011?�Ͼ�ģ�⣩2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������InGaN���������أ�Ϊ�����黯�ص�Ʒ���ṹ��ͼ���Իش�
��2011?�Ͼ�ģ�⣩2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������InGaN���������أ�Ϊ�����黯�ص�Ʒ���ṹ��ͼ���Իش�