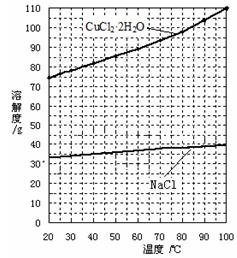
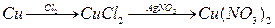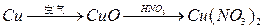��Ŀ����
������ҵ�ǹ��ҹ�ҵ�Ļ�������ش𣺸���ұ������ʴ����������е��й����⡣
��1����ҵ���Ȼ�ԭ��ұ����������Ҫԭ���� ���豸�������� д���û�ԭ��CO��ԭ��������Ҫ�ɷ�ΪFe2O3���Ļ�ѧ����ʽ
��
��2��д�������ڳ�ʪ�����з�����ʴʱ��������Ӧ����ʽ
�������⣨Fe2O3..XH2O���Ļ�ѧ����ʽΪ .
(3)��������;ԶԶ�����ֲĹ㷺�����ǰѽ϶�ĸ�¯����ֱ��ұ���ɸ֣�������ֱ���ڴ�������ת¯��ת���ɸ�ʱ���ǰ��к����ʳ�������������Ԫ�أ����Ը���Ϊ
��Ϊ�˷�ֹ������Ʒ������ʴ�������ڸ�������ı�����е��ͭ�ȴ�ʩ�����ͭʱ��������ӦΪ ��
��1����ҵ���Ȼ�ԭ��ұ����������Ҫԭ���� ���豸�������� д���û�ԭ��CO��ԭ��������Ҫ�ɷ�ΪFe2O3���Ļ�ѧ����ʽ
��
��2��д�������ڳ�ʪ�����з�����ʴʱ��������Ӧ����ʽ
�������⣨Fe2O3..XH2O���Ļ�ѧ����ʽΪ .
(3)��������;ԶԶ�����ֲĹ㷺�����ǰѽ϶�ĸ�¯����ֱ��ұ���ɸ֣�������ֱ���ڴ�������ת¯��ת���ɸ�ʱ���ǰ��к����ʳ�������������Ԫ�أ����Ը���Ϊ
��Ϊ�˷�ֹ������Ʒ������ʴ�������ڸ�������ı�����е��ͭ�ȴ�ʩ�����ͭʱ��������ӦΪ ��
��1������ʯ����̿��ʯ��ʯ ��¯
Fe2O3+3CO="2" Fe+3CO2
(2)O2+2H2O+4e-=4OH- 4 Fe+3O2+2XH2O="2" Fe2O3.XH2O
(3)��̼�������ף������������� Cu-2e-=Cu2+
Fe2O3+3CO="2" Fe+3CO2
(2)O2+2H2O+4e-=4OH- 4 Fe+3O2+2XH2O="2" Fe2O3.XH2O
(3)��̼�������ף������������� Cu-2e-=Cu2+
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
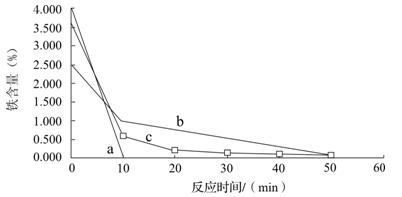
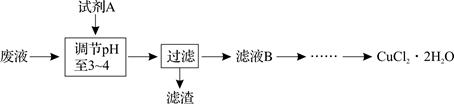
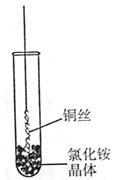
 ����ͭ
����ͭ ����ͭ
����ͭ