��Ŀ����
����Ŀ�����ſ�ѧ�����ķ�չ�������ӵ������IJⶨ�ֶ�Խ��Խ�࣬�ⶨ��ȷ��ҲԽ��Խ�ߣ�����һ�ּ��еIJⶨ���������岽��Ϊ��
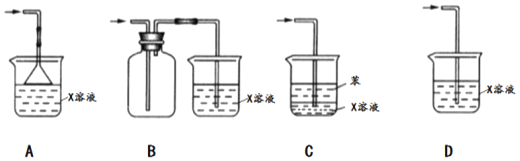
(1)������NaCl��ϸ�������ȷ��ȡmgNaCl���岢ת�Ƶ���������A�С�
(2)�õζ�����A�����еμӱ��������������ӱ���A�����Ŀ̶��ߣ������NaCl��������ΪVcm3��
�ٲ���(1)��A���������__________���������ƣ���
�ڲ���(2)������ʽ�ζ��ܺû��Ǽ�ʽ�ζ��ܺã�__________��������______________��
���ܷ��ý�ͷ�ιܴ��沽��(2)�еĵζ���__________��������____________________��
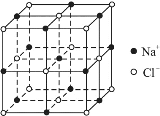
����֪NaCl����Ľṹ����ͼ��ʾ����X���߲��NaCl�����п��������Na+��Cl-���ƽ������Ϊacm�����������ⶨ������ð����ӵ�����NA�ı���ʽΪ��NA=______mol-1��
���𰸡�����ƿ ��ʽ�ζ��� �����ܽ��ʽ�ζ��ܵ���Ƥ�� ���� ʵ������Ҫȷ��ȡ������� ![]()
��������
������ƿΪ�����������ܹ���ȷ�IJⶨ�����
�ڱ�Ӧ����ʽ�ζ���ʢװ�����ܸ�ʴ��ʽ�ζ��ܵ���Ƥ�ܣ�
��ʵ������Ҫȷ��ȡ�����������˲����ý�ͷ�ιܴ���ζ��ܣ�
����ʵ����NaCl����������������Եõ�NaCl������ܶȣ���̯�����㾧����Na+��Cl-��Ŀ���ð���٤��������ʾ�����������������Na+��Cl-���ƽ������Ϊacm�����ⳤ=2acm���������������ھ����ܶ��뾧������˻����������㡣
���ܹ���ȷ�IJⶨNaCl������������Բ���(1)���������������ƿ��
�����ڱ����������͡��ϻ����ã����Եζ�������ʽ�ζ��ܣ�
��ʵ������Ҫȷ��ȡ�����������˲����ý�ͷ�ιܴ���ζ��ܣ�
��NaCl���ܶ�Ϊ��=![]() g/cm3�������Na+��Cl-���ƽ������Ϊacm�����ⳤ=2acm��NaCl���������=(2a)3cm3��������Na+������Ŀ=1+12��
g/cm3�������Na+��Cl-���ƽ������Ϊacm�����ⳤ=2acm��NaCl���������=(2a)3cm3��������Na+������Ŀ=1+12��![]() ��Cl-��Ŀ=8��
��Cl-��Ŀ=8��![]() +6��
+6��![]() =4����NaCl����������=
=4����NaCl����������=![]() g=
g=![]() g/cm3��(2a)3cm3�������ɵ�NA=
g/cm3��(2a)3cm3�������ɵ�NA=![]() ��
��

����Ŀ���ϳɰ��������ѧ������չʷ�ϵ�һ���ش�ͻ�ƣ��о�����Һ����һ�����õĴ������ʡ�
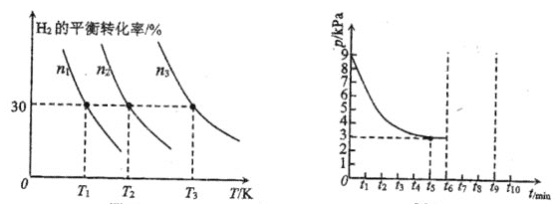
(1)N2(g)+3H2(g)2NH3(g) ��H��0���о��������������ɼ��ٰ����ĺϳɡ��±�Ϊij�¶��µ������IJ�ͬ�����ֱ���ϳɰ���ʱ�������ij�ʼ����(mmol min-1)��
���� | Ru | Rh | Ni | Pt | Pd | Fe |
��ʼ���� | 7.9 | 4.0 | 3.0 | 2.2 | 1.8 | 0.5 |
�ٲ�ͬ���������£��ϳɰ�����Ӧ�Ļ��������_______ (��д�����Ļ�ѧʽ)��
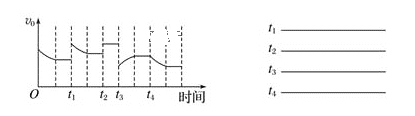
��ij�о�С���һ��̽�������Ժϳɰ�����Ӱ�졣��N2��H2��һ��������ͨ�����ֲ�ͬ�Ĵ������з�Ӧ����ͬʱ���ڲ����ݳ�������NH3����������ͼ���Ӷ�ȷ���¶ȶԴ�����Ӱ�졣a��___ (��ǡ����ǡ�)��Ӧ�¶��°���ƽ��ʱ�İٷֺ�����˵��������___��

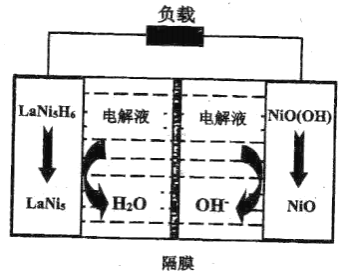
(2)�����ķֽⷴӦ2NH3N2+3H2 ��H��0������ʵ��������������������ͬʱͨ�백����ˮ���������������ѹ�Ͱ���ת������ʱ��仯�����ͼ��ʾ��
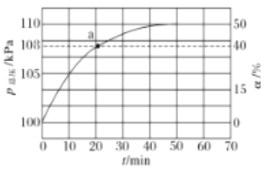
��ƽ��ʱ��p(H2O)= ___kPa��ƽ�ⳣ��Kp=_____KPa2(KpΪ�Է�ѹ��ʾ��ƽ�ⳣ��)��
�ڷ�Ӧ����v=v��-v��=K��p2(NH3)-K��p(N2)p3(H2)��k����k���ֱ�Ϊ�����淴Ӧ���ʳ���������a����![]() =__��
=__��
(3)Һ���У�2NH3(l)NH2-+NH4+����Pt�缫��Һ�����е��Ҳ�ɲ���H2��N2�������ĵ缫��Ӧ______��
(4)���������ð�ˮ����SO2������SO2������ͨ��ð�ˮ�У�����Һ������ʱ����Һ�е�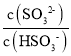 =____��(��֪25�棬Kb(NH3H2O)=1.810-5��Ka1(H2SO3)=.310-2��Ka2(H2SO3)=6.210-8)
=____��(��֪25�棬Kb(NH3H2O)=1.810-5��Ka1(H2SO3)=.310-2��Ka2(H2SO3)=6.210-8)
����Ŀ����������茶���[(NH4)2Fe(SO4)2��6H2O]��һ���ȶ��Ļ�ԭ����������������ѧ�еĵζ�����ij������������з����Ʊ��þ��壬���������ȶ��Խ���������ʵ�顣
��.��������茶�����Ʊ�:
����![]() FeSO4��Һ
FeSO4��Һ![]() �ᾧ
�ᾧ![]() ��������茶���
��������茶���
(1)����Ũ�ȹ���Ӧ����ͬʱ��ʹFeSO4��Һ�л���_____________(�����ӷ���)��
(2)����FeSO4��Һ��(NH4)2SO4�����Ʊ��þ���Ļ�ѧ����ʽ_____________��
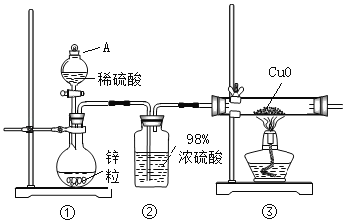
��.��������茶�����ȷֽ����̽��:��С��ͬѧѡ����ͼ��ʾ����װ�ý���ʵ��(�г�װ����)����������:��������������500���������������ȫ�ֽ⣬�����к�����������������������ˮ�����ȡ�
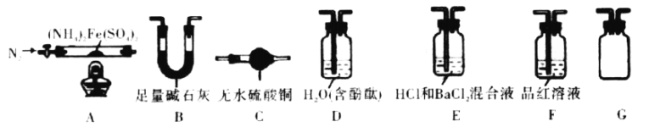
(3)��ȼA���ľƾ���֮ǰ���ȴ��ɼУ�ͨһ��ʱ��N2����Ŀ����____________��
(4)ѡ���������ֱ�Ҫ��װ�����������Ӧʵ�飬��д�����ϵ����ݡ�
������ܲ��� | װ������˳�� | װ������ | ʵ������ | ʵ����� |
H2O��NH3 | ACBGD | ��B������______ | ��C��________�� D��__________�� | ��H2O��NH3 |
SO2��SO3 | ��___________ | ��E��HCl������ ___________ | E��û����������F����Һ��ɫ | ��___________ |
(5)Ϊ֤���ȷֽ���ȫ������Ĺ����ΪFe2O3��������FeO��Fe3O4����Ҫѡ�õ��Լ���__________(ѡ������ĸ)������������ѡ�Լ�����ʵ��֤���ķ���____________��
A.ϡ���� B.ϡ���� C.KSCN��Һ D.H2O2��Һ E.K3[Fe(CN)6]��Һ