��Ŀ����
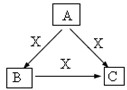 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��������������ֲ�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��������������ֲ�ͬ����ش���1����A��B��C�о���ͬһ�ֳ�������Ԫ�أ���Ԫ����C������������ʽ���ڣ���A��C��ˮ��Һ��Ͽɵ�B�İ�ɫ��״������
��A�к��еĽ���Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
�ڸý���Ԫ�صĵ�����ij��ɫ�������ڸ����·�Ӧ�������ں������켰�����ƣ���֪��1mol�õ�����ȫ��Ӧ�����¶Ȼָ���298Kʱ��������Q kJ����д���÷�Ӧ���Ȼ�ѧ��Ӧ����ʽΪ
��2����AΪ�л��75%��A��Һ����Ϊ�����������³�ѹ��B��C��Ϊ��ɫ���壬C��һ�ֳ�������������A�Ľṹ��ʽΪ��
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��ˮ��Һ��Ϊ���ԣ�
���û�ѧ����ʽ����C��Һ�ʼ��Ե�ԭ��
�ڽ�4.48L����״���£�Xͨ��100mL3mol/L A��ˮ��Һ����Һ������Ũ���ɴ�С��˳��Ϊ
����Ȼ���д���B��C��H2O��һ�������ᾧ���ɵĹ��壮ȡһ�����ù�������ˮ���100mL��Һ�������Һ�н��������ӵ�Ũ��Ϊ0.5mol/L����ȡ��ͬ�����Ĺ�����������أ�ʣ����������Ϊ
������A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�
��1����A��B��C�о���ͬһ�ֳ�������Ԫ�أ���Ԫ����C������������ʽ���ڣ���ת����ϵ������CΪAlO2-����A��C��ˮ��Һ��Ͽɵ�B�İ�ɫ��״��������֪A���������ӣ�BΪ����������C����ƫ�������xΪ�������ƣ�����ת����ϵ��
��2����AΪ�л��75%��A��Һ����Ϊ����������AΪCH3CH2OH�����³�ѹ��B��C��Ϊ��ɫ���壬C��һ�ֳ�����������ӦΪCO2����BΪCO��XΪO2��
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��������Ԫ�أ�ˮ��Һ��Ϊ���ԣ���ת����ϵ��AΪ�������ƣ�BΪ̼���ƣ�CΪ̼�����ƣ�xΪ������̼��
��1����A��B��C�о���ͬһ�ֳ�������Ԫ�أ���Ԫ����C������������ʽ���ڣ���ת����ϵ������CΪAlO2-����A��C��ˮ��Һ��Ͽɵ�B�İ�ɫ��״��������֪A���������ӣ�BΪ����������C����ƫ�������xΪ�������ƣ�����ת����ϵ��
��2����AΪ�л��75%��A��Һ����Ϊ����������AΪCH3CH2OH�����³�ѹ��B��C��Ϊ��ɫ���壬C��һ�ֳ�����������ӦΪCO2����BΪCO��XΪO2��
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��������Ԫ�أ�ˮ��Һ��Ϊ���ԣ���ת����ϵ��AΪ�������ƣ�BΪ̼���ƣ�CΪ̼�����ƣ�xΪ������̼��
����⣺��1����A��B��C�о���ͬһ�ֳ�������Ԫ�أ���Ԫ����C������������ʽ���ڣ���ת����ϵ��֪��A���������ӣ�BΪ����������C����ƫ�������xΪ�������ƣ�A��B��C�к��е�ͬһ�ֳ�������Ԫ��ΪAl��
��A�к��еĽ���ΪAl��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ3����ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��Al���������������ȷ�Ӧ�����ں������켰�����ƣ��˷�Ӧ�Ļ�ѧ����ʽΪ2Al+Fe2O3
Al2O3+2Fe��1molAl��ȫ��Ӧ�����¶Ȼָ���298Kʱ��������Q kJ��
��Ӧ���Ȼ�ѧ����ʽΪ2Al��s��+Fe2O3��s��
Al2O3��s��+2Fe��s������H=-2QkJ/mol��
�ʴ�Ϊ��2Al��s��+Fe2O3��s��
Al2O3��s��+2Fe��s������H=-2QkJ/mol��
��2����AΪ�л��75%��A��Һ����Ϊ����������AΪCH3CH2OH�����³�ѹ��B��C��Ϊ��ɫ���壬C��һ�ֳ�����������ӦΪCO2����BΪCO��XΪO2��
��CO��O2�ֱ�ͨ����CH3CH2OH�Ƴɵ�����缫����20%-30%��KOH��Һ��Ϊ�������Һ��������ɻ�ѧ��Դ���õ�طŵ�ʱ�������缫��ӦʽΪ2CO+8OH--4e-=2CO32-+4H2O��
�ʴ�Ϊ��CH3CH2OH��2CO+8OH--4e-=2CO32-+4H2O��
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��������Ԫ�أ�ˮ��Һ��Ϊ���ԣ���ת����ϵ��AΪ�������ƣ�BΪ̼���ƣ�CΪ̼�����ƣ�xΪ������̼��
��CΪ̼�����ƣ�Ϊǿ�������Σ�ˮ��ʼ��ԣ�����ʽΪCO32-+H2O OH-+HCO3-���ʴ�Ϊ��CO32-+H2O
OH-+HCO3-���ʴ�Ϊ��CO32-+H2O OH-+HCO3-��
OH-+HCO3-��
��4.48L����״���£�CO2�����ʵ���Ϊ
=0.2mol��100mL3mol/L NaOH��ˮ��Һ��n��NaOH��=0.1L��3mol/L=0.3mol��n��CO2����n��NaOH��=0.2mol��0.3mol=2��3=1��1.5������1��1��1��2֮�䣬�ʷ�Ӧ����̼������̼�����ƣ���̼������̼�����Ƶ����ʵ����ֱ�Ϊamol��bmol�����������غ���2a+b=0.3����̼Ԫ���غ���a+b=0.2���������̣����a=0.1��b=0.1��̼�����̼�����ˮ�⣬��Һ�ʼ���c��OH-����c��H+����̼�����ˮ��̶ȱ�̼������Ĵ�c��HCO3-����c��CO32-����ˮ��̶Ⱥ�С��c��CO32-��Զ����c��OH-������Һ���������Ѷ���ʣ�c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
����Ȼ���д���B��C��H2O��һ�������ᾧ���ɵĹ��壮ȡһ�����ù�������ˮ���100mL��Һ����������н��������ӵ�Ũ��Ϊ0.5mol/L����������Ũ��Ϊ0.5mol/L��ȡ��ͬ�����Ĺ�����������أ�ʣ�����Ϊ̼���ƣ������������غ��֪��̼���Ƶ�����Ϊ
��0.1L��0.5mol/L��106g/mol=2.65g��
�ʴ�Ϊ��2.65 g��
��A�к��еĽ���ΪAl��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ3����ԭ�ӽṹʾ��ͼΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ��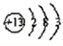 ��
����Al���������������ȷ�Ӧ�����ں������켰�����ƣ��˷�Ӧ�Ļ�ѧ����ʽΪ2Al+Fe2O3
| ||
��Ӧ���Ȼ�ѧ����ʽΪ2Al��s��+Fe2O3��s��
| ||
�ʴ�Ϊ��2Al��s��+Fe2O3��s��
| ||
��2����AΪ�л��75%��A��Һ����Ϊ����������AΪCH3CH2OH�����³�ѹ��B��C��Ϊ��ɫ���壬C��һ�ֳ�����������ӦΪCO2����BΪCO��XΪO2��
��CO��O2�ֱ�ͨ����CH3CH2OH�Ƴɵ�����缫����20%-30%��KOH��Һ��Ϊ�������Һ��������ɻ�ѧ��Դ���õ�طŵ�ʱ�������缫��ӦʽΪ2CO+8OH--4e-=2CO32-+4H2O��
�ʴ�Ϊ��CH3CH2OH��2CO+8OH--4e-=2CO32-+4H2O��
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��������Ԫ�أ�ˮ��Һ��Ϊ���ԣ���ת����ϵ��AΪ�������ƣ�BΪ̼���ƣ�CΪ̼�����ƣ�xΪ������̼��
��CΪ̼�����ƣ�Ϊǿ�������Σ�ˮ��ʼ��ԣ�����ʽΪCO32-+H2O
 OH-+HCO3-���ʴ�Ϊ��CO32-+H2O
OH-+HCO3-���ʴ�Ϊ��CO32-+H2O OH-+HCO3-��
OH-+HCO3-����4.48L����״���£�CO2�����ʵ���Ϊ
| 4.48L |
| 22.4L/mol |
�ʴ�Ϊ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
����Ȼ���д���B��C��H2O��һ�������ᾧ���ɵĹ��壮ȡһ�����ù�������ˮ���100mL��Һ����������н��������ӵ�Ũ��Ϊ0.5mol/L����������Ũ��Ϊ0.5mol/L��ȡ��ͬ�����Ĺ�����������أ�ʣ�����Ϊ̼���ƣ������������غ��֪��̼���Ƶ�����Ϊ
| 1 |
| 2 |
�ʴ�Ϊ��2.65 g��
���������⿼��������ƶϣ�Ϊ�߿��������ͣ�������ѧ���ķ���������Ԫ�ػ�����֪ʶ���ۺ����õĿ��飬��ϤԪ�ػ�����֪ʶ�ǽ���ǰ�ᣬ��Ŀ�Ѷ��еȣ�ע��BΪ��ɫ��������3������ɫΪͻ�ƿڣ����ó���Ԫ�ػ���������ʽ��ת����ϵѡ����ʵ����ʽ��н�ɣ�

��ϰ��ϵ�д�
�����Ŀ
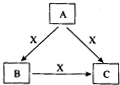 A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺
A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺ A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�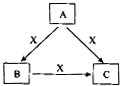 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��������������ֲ�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��������������ֲ�ͬ����ش�
 A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺ A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺