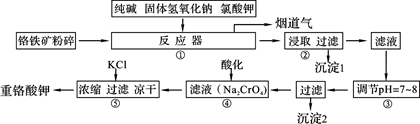
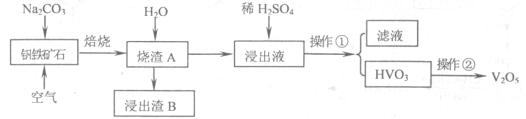
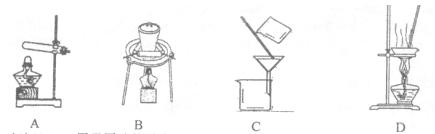
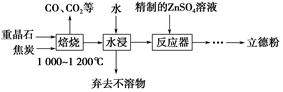
��Ŀ����
�Ȼ�識�ơ���李����ֳ�±ɰ��Ϊ��ɫ������ɫ�ᾧ�Է�ĩ��������ˮ�У��ڹ�ũҵ��������;�㷺�����Ȼ��ƺ������Ϊԭ���Ʊ��Ȼ�識�����Ʒ�����ƣ������������£�
�Ȼ�狀������Ƶ��ܽ�����¶ȱ仯��ͼ��ʾ���ش��������⣺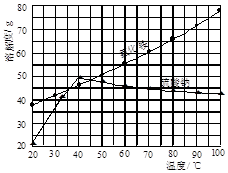
��1��ʵ���ҽ�������Ũ���õ�����Ҫ������ ���ձ������������ƾ��Ƶȡ�
��2��ʵ������г��ȹ��˵�Ŀ���� ��
��3��д��������Ũ����ʱ�����Ļ�ѧ����ʽ�� ��
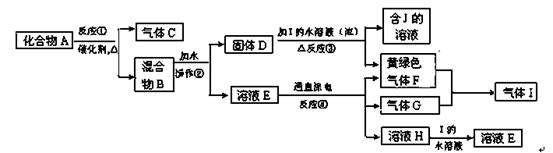
��4��ij�о���ѧϰС��Ϊ�ⶨ��NH4Cl��Ʒ�е��ĺ������������ͼװ�ã������������ۡ�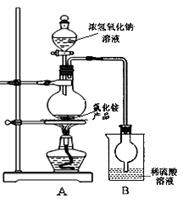
��ͬѧ�����ݴ�ʵ���õ����ݣ������NH4Cl��Ʒ�ĺ���������ƫ�ߣ���Ϊʵ��װ���д���һ������ȱ���ǣ� ____ ��
��ͬѧ��ʵ������У�����ƿ�м����Ũ����������Һ�����ӷ�Ӧ����ʽΪ ����Ӧ������NaOHһ��Ҫ��������ּ��ȣ�ԭ���� ��
�øĽ����ʵ��װ�����½���ʵ�飬��ȡ13.0gNH4Cl��Ʒ�����ʵ���Bװ������3.4g����û��ʺ�����Ϊ ��
��1��������
��2����ֹ�Ȼ�茶������������
��3����NH4��2SO4��2NaCl= Na2SO4����2NH4Cl
��4����A��Bװ�ü�ȱһ������װ�� �� NH4++OH- NH3��+H2O
NH3��+H2O
ʹ�Ȼ�麟�ַ�Ӧ��ȫת��ΪNH3 21.5��
���������������1��ʵ���ҽ�������Ũ���õ�����Ҫ�������������ձ������������ƾ��Ƶȣ� ����
��2����ͼ����Կ�������ijһ�¶ȷ�Χ�ڣ��Ȼ�淋��ܽ�ȵ�������淋��ܽ�ȣ����Գ��ȹ��˷�ֹ�Ȼ�茶�����������ģ�����ʹ������Գ�����ʽ�˳����Ȼ��������Һ�
��3������Ũ��ʱ������Թ�����ʽ���ڣ����Ի�ѧ����ʽΪ��NH4��2SO4��2NaCl= Na2SO4����2NH4Cl
��4���ٰ����ڽ���B֮ǰδ������Ժ�����ƫ�ߣ�Ӧ��A��Bװ�ü��һ������װ�ã���
���㣺���黯ѧ�빤ҵ��������ϵ����ʵ��ķ�����ʵ��װ�õ��жϣ����ӷ���ʽ����д����ѧ�����

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д� ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д��������̿������ɵ��ȥ�������ϳɹ�ҵ�Ĵ�������������������ҵ���´ɹ�ҵ����ɫ������ɫ�����������ȡ�
��1���������������Խ�������һ��ǿ�����������û�ѧ����ʽ֤����______________________��
��2��п���̼��Ե�ؾ��������ŵ��������ص㣬����õ��㷺Ӧ�á���ص��ܷ�ӦʽΪZn��s����2MnO2��s����H2O��l��=Zn��OH��2��s����Mn2O3��s����
�ٵ�ع���ʱ��MnO2����________��Ӧ��
�ڵ�ص�������ӦʽΪ________��
��3����ҵ�������̿�Ϊԭ�ϣ��������������Ʊ��ߴ��������̵��������£�
��֪�����̿����Ҫ�ɷ�ΪMnO2������Si��16.27%����Fe��5.86%����Al��3.42%����Zn��2.68%����Cu��0.86%����Ԫ�صĻ��������������������������������ʽ��ȫ����ʱ��Һ��pH���±���
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Mn��OH��2 | Cu��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 10.4 | 6.7 |
| ������ | Zn��OH��2 | CuS | ZnS | MnS | FeS |
| pH | 8.0 | ��0.42 | 2.5 | 7 | 7 |
�ش��������⣺
���������������������½�MnO2��ԭΪMnSO4�����ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ________________________��
���Լ�XΪ________��
������A����Ҫ�ɷ�Ϊ________��
�ܼ���MnS��Ŀ����Ҫ�dz�ȥ��Һ�е�________��
��ҵ���þ���þ��(����MgO��KCl��MgCl2��BaCl2��CaCl2��FeCl3������)����MgCl2�Ĺ�ҵ��������:
��֪:25 ��ʱ�й����ʵ��ܶȻ�����:
| ���� | CaCO3 | MgCO3 | BaCO3 | Mg(OH)2 | Fe(OH)3 |
| Ksp | 4.96��10-9 | 6.82��10-6 | 5.1��10-9 | 5.61��10-12 | 2.64��10-38 |
(1)д���ܽ�ʱ�����ӷ���ʽ ��
(2)�ܽ�ʱ�¶Ȳ���̫��,Ҳ����̫��,Ҫ�������35 ������,�������� ��
(3)����������������,�ֱ�Ϊ ,����,ϴ��,��ɡ����ʱ��Ҫ��ѹ���,ԭ���� ��
(4)Ϊ����Na2CO3����������߲�Ʒ����,���к���(�кͺ���Һ�ӽ�����)����ǰҪ������Һ���Ƿ���� ����,ѡ���������ӵ�ԭ���� ��
(5)ĸҺ����Ҫ�ɷ� ��
���ú��̷�ˮ����Ҫ��Mn2+��SO42-��H+��Fe2+��Al3+��Cu2+�����Ʊ������ܴ��Բ���̼���̣�MnCO3��������һ�ֹ�ҵ�������£�
��֪ijЩ������ȫ������pHֵ���±���
| ������ | Fe(OH)3 | Al(OH)3 | Cu(OH)2 | Mn(OH)2 | CuS | MnS | MnCO3 |
| ������ȫʱ��PH | 3.7 | 5.2 | 6.4 | 9.8 | ��0 | ��7 | ��7 |
��1�����������ʾ��ܰ�Fe2+����ΪFe3+������̢��п�ѡ�������������� ��
a��Cl2 b��MnO2 c��ŨHNO3 d��H2O2
��2�����̢��У����������ijɷ��� ��
��3�����̢��У������Ŀ���� ��������Ӧ�����ӷ���ʽ�� ��
��4�����̢��У������ɵ�����J��ʹ����ʯ��ˮ����ǣ�������MnCO3��Ӧ�����ӷ���ʽ��___________��
��5����MnCO3���Ƶ���Ҫ�Ĵ���MnO2��MnCO3 +
 O2 �� MnO2 + CO2��
O2 �� MnO2 + CO2�����ڿ����м��� 460.0 g��MnCO3���õ�332.0 g��Ʒ������Ʒ������ֻ��MnO����ò�Ʒ��MnO2������������ ����Ħ������/g����MnCO3 115 MnO2 87 MnO 71��
 BaS��s����4CO2��g�����÷�Ӧ��ƽ�ⳣ���ı���ʽΪ__________________________________________________��
BaS��s����4CO2��g�����÷�Ӧ��ƽ�ⳣ���ı���ʽΪ__________________________________________________��