��Ŀ����
��11�֣���ⷨ���ռ��ԭ���DZ���ʳ��ˮ�����ڴ����к�����ɳ��Ca2+��Mg2+��Fe3+��SO42-���ʣ������ϵ��Ҫ����˱��뾭�����ơ�ijУʵ��С�龫�ƴ���ˮ��ʵ��������£�
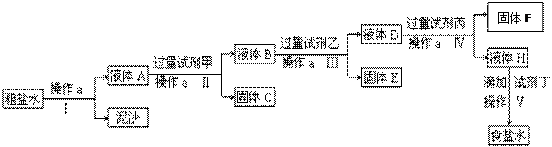
��ش��������⣺
��1������a��������____________��
��2���ڢ��У���������Լ������������ִ����ij��������Լ���Ϊ________��Һ���Լ���Ϊ ������FΪ ��
��3���ڵڢ����У������Լ���ֱ����Һ�����Ա仯ʱ��д���˹��̵Ļ�ѧ����ʽ �� _ ��

��ش��������⣺
��1������a��������____________��
��2���ڢ��У���������Լ������������ִ����ij��������Լ���Ϊ________��Һ���Լ���Ϊ ������FΪ ��
��3���ڵڢ����У������Լ���ֱ����Һ�����Ա仯ʱ��д���˹��̵Ļ�ѧ����ʽ �� _ ��
��1�����ˣ�1�֣���2��NaOH��BaCl2��CaCO3��BaCO3��ÿ��2�֣���3��NaOH + HCl =" NaCl" + H2O Na2CO3 + 2HCl =" 2NaCl" + H2O + CO2����ÿ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
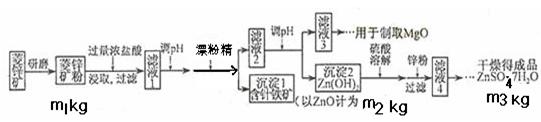
 ���½�һ�����᳧������Ϊ��ַ��ѡ�� �Ľ�������ѡ��ı�ţ�
���½�һ�����᳧������Ϊ��ַ��ѡ�� �Ľ�������ѡ��ı�ţ�
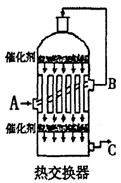
 2ZnO��2SO2 2C��O2
2ZnO��2SO2 2C��O2
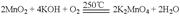 ��
��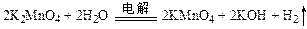 ��
��