��Ŀ����
��һδ֪����ɫ��Һ��ֻ���ܺ������������е������֣�������ˮ���������H+��OH-����H+��NH4+��K����Mg2����Cu2����Al3+��NO3����CO32����SO42������ȡ����100mL��Һ��������ʵ�飺
�ٵ�һ�ݼ�����AgNO3��Һ���а�ɫ����������
�ڵڶ��ݼ�����BaCl2��Һ���а�ɫ������������ϴ�ӡ������������Ϊ6.99 g��
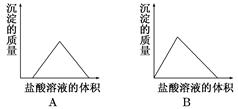
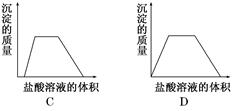
�۵�������εμ�NaOH��Һ����ó�����NaOH��Һ�������ϵ��ͼ����������ʵ�飬�����Ʋⲻ��ȷ����
| A��ԭ��Һһ��������H+��Cu2����CO32�� |
| B������ȷ��ԭ��Һ�Ƿ���K����NO3�� |
| C��ԭ��Һȷ����Mg2����Al3����NH4������n(Mg2��):n(Al3��):n( NH4��)��1:1:2 |
| D��ʵ�����ӵ�NaOH��Ũ��Ϊ2mol��L�� |
B
���������������Һ����ɫ�ģ����һ��������ͭ���ӡ���һ�ݼ�����AgNO3��Һ���а�ɫ������������˵����Һ�����ٺ���CO32����SO42���е�һ�֡��ڶ��ݼ�����BaCl2��Һ���а�ɫ������������ϴ�ӡ������������Ϊ6.99 g����ð��´������ᱵ��̼�ᱵ���Ƕ��ߵĻ�����������εμ�NaOH��Һ����ó�����NaOH��Һ�������ϵ��ͼ������ͼ���֪����ʼ�������ֳ�������˵�������ڴ����������ӡ��������ﵽ���ֵʱ���ֲ��䣬��˵�������������������Һ�е�NH4����Ӧ����һˮ�ϰ������һ������NH4����������������������Һ��������ʼ���٣�������ȫ��ʧ����˵������Ӧ����������þ�����������Ļ���������Һ��һ������Mg2����Al3������һ��������CO32��������һ��������SO42����A��ԭ��Һһ��������H����Cu2����CO32����A��ȷ��B�����ᱵ��������6.99g�����ʵ�����6.99g��233g��mol��1��0.03mol������ͼ���֪��������þ�����ʵ�����0.01mol�����ʱ������þ���ĵ���������Ӧ����0.02mol���ܽ������������ĵ�����������Һ�����5ml������������������ĵ�����������Һ���15ml�����Բ���������þ���ĵ�����������Һ�����10mol�����Ը��ݷ���ʽMg2����2OH����Mg(OH)2����Al3����3OH����Al(OH)3����֪��������þ���������������ʵ�����ȣ�����0.01mol����NH4����Ӧ������������Һ�����10ml������NH4�������ʵ�����0.02mol��������Һ�ĵ����Կ�֪����Һ��������SO42���ĵ������0.06mol���������� �ĵ������0.02mol+0.03mol+0.02mol��0.07mol��������Һ��һ��������NO3����������ȷ��ԭ��Һ�Ƿ���K����B����ȷ��C��ԭ��Һȷ����Mg2����Al3����NH4������n(Mg2��):n(Al3��):n( NH4��)��1:1:2��C��ȷ��D��ʵ�����ӵ�NaOH��Ũ��Ϊ0.02mol��0.01L��2mol��L��1��D��ȷ����ѡB��
���㣺�������ӹ��桢���Ӽ�����й��жϺͼ���

�±������ۺ�������
| ѡ�� | ���� | ���� |
| A | һ��������0.5mol N2��1.5molH2��Ӧ��ƽ��ʱ����akJ����N2(g)+3H2(g) 2NH3(g) 2NH3(g) 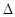 H=2akJ��mol-1 H=2akJ��mol-1 | ��ȷ |
| B | �Ȼ�þ��Һ�백ˮ��Ӧ�� Mg2++2OH-=Mg(OH)2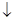 | ����ˮ��Ӧд��������ʽ |
| C | ���������Һ�м�������������Һ�� Ba2++SO42-=BaSO4 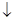 | ��ȷ |
| D | �����ʵ�����Cl2��FeBr2����Һ�з�Ӧ�� 2Fe2++2Br-+2C12==2Fe3++4Cl-+Br2 | ����Fe2+��Br-�Ļ�ѧ������֮��ӦΪl��2 |
�������ӷ���ʽH+ + OH- = H2O��ʾ����
| A��Ba(OH)2��Һ��H2SO4��Һ��� |
| B��NaOH��Һ�������� |
| C��Cu(OH)2��ϡH2SO4��Ӧ |
| D��CO2ͨ��NaOH��Һ�� |
���и��������У��ڸ����������ܹ������������( )
| A��ͨ������CO2�����Һ�У�Na+��SiO32����CH3COO����CO32�� |
| B����ɫ��Һ�У�Mg2+��MnO4����SO42����K+ |
| C��c(H+)/c(OH��)=1012����Һ�У�NH4+��Al3+��NO3����Cl�� |
| D��c(ClO��)=" 1.0" mol/L����Һ��Na+��SO32����S2����SO42�� |
�������ӷ���ʽ��д��ȷ����
| A�������[NH4Al(SO4)2��12H2O]��Һ�м������Ba(OH)2��Һ��Al3+��2SO42����2Ba2+��4OH����AlO2����2BaSO4����2H2O |
| B��H218O��Ͷ��Na2O2���壺2H218O + 2Na2O2 = 4Na+ + 4OH- + 18O2�� |
| C��̼�������Һ�мӹ�������ʯ��ˮ��Ca2+ + OH- + HCO3- = CaCO3�� + H2O |
D��̼���Ƶ�ˮ�ⷴӦ��CO32��+ H3O�� HCO3��+ H2O HCO3��+ H2O |
����ȷ��ʾ���з�Ӧ�����ӷ�Ӧ����ʽΪ��������
A�����Ե缫���MgCl2��Һ��2Cl- +2 H2O 2OH- +Cl2�� +H2�� 2OH- +Cl2�� +H2�� |
| B����������Һ�еμ�Ba(OH)2��Һ��ǡ��ʹSO42-������ȫ��2Al3++3SO42-+3Ba2++6OH -="2" Al(OH)3��+3BaSO4�� |
C��HS-�ĵ��룺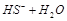   |
| D�������ȥˮ����2H++CaCO3=Ca2++ CO2��+ H2O |
����˼ά�ǻ�ѧ�����г��õ�һ��˼ά�����������йط�Ӧ����ʽ��������ȷ���ǣ� ��
| | ��֪ | ���� |
| A | ��Fe����CuSO4��Һ�У�Fe��Cu2��=Cu��Fe2�� | ��Na���뵽CuSO4��Һ�У�2Na��Cu2��=Cu��2Na�� |
| B | ϡ������Ba��OH��2��Һ��Ӧ�����ԣ�2H����SO42-��Ba2����2OH��=BaSO4�� ��2H2O | NaHSO4��Һ��Ba��OH��2��Һ��Ӧ�����ԣ�2H����SO42-��Ba2����2OH��=BaSO4����2H2O |
| C | ����������Ӧ��2Fe��3Cl2 2FeCl3 2FeCl3 | ���͵ⵥ�ʷ�Ӧ��2Fe��3I2 2FeI3 2FeI3 |
| D | ��Ca��ClO��2��Һ��ͨ������CO2��Ca2����2ClO����CO2��H2O=CaCO3����2HclO | ��Ca��ClO��2��Һ��ͨ������SO2��Ca2����2ClO����SO2��H2O=CaSO3����2HClO |