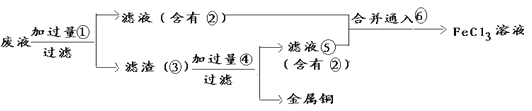
��Ŀ����
����Ŀ����ͼ��ijѧ����Ƶ�ʵ�����Ʊ�����Cl2�����ն���������ʵ��װ��ͼ����ش�
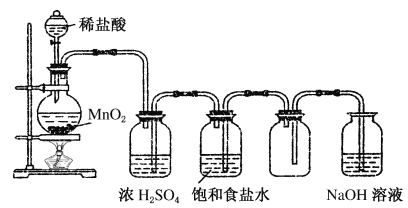
(1)ָ������ͼ�еĸ�������
��_________________________________________________________��
��_________________________________________________________��
(2)�ڸĹ����װ���У��������ʵ����÷ֱ��ǣ�
�ٱ���ʳ��ˮ_________________________________________��
��Ũ����____________________________________________��
��NaOH��Һ_________________________________________��
(3)��ͬѧ���������ͼ��ʾ��ʵ��װ�ò�����ʵ�飺

�ټ�ͬѧʵ���û�еõ�Ԥ�ڵ�ʵ�������������������ʵ��ʧ�ܵ�ԭ��_____________________��
�ڼ�ͬѧ��ʵ��ʧ�ܺ����Ƶ�ʵ��װ�ý����˸Ľ��������½�����ʵ�飬����õ���Ԥ�ڵ�ʵ����������Ϊ��Ԥ�ڵ�ʵ��������______________________________________���ɴ˵ó�Cl2ʹ��ɫ������ɫ�Ļ�����________________________________________��
(4)�������������������20mL12mol/L��Ũ�����ϼ��ȣ���ַ�Ӧ�����ɵ�������������0.06mol������Ҫԭ���Т�_______________________����_____________________________��
(5)Ϊ�����Ũ����������ʣ���Ը�ʵ��Ľ�����___________________________________��
(6)д��Բ����ƿ�з�����Ӧ�����ӷ���ʽ__________________________________________��
ʵ�������ϴ����ʱ��Ϊ�˼�����ƿ�в��������Ի�������Ⱦ����������ƿ�м������Һ��_________���йص����ӷ���ʽ��______________________________________��
���𰸡�������ϡ���ᣬ��Ӧ��Ũ����ʢŨ�����ʢ����ʳ��ˮ��ϴ��ƿλ�õߵ���ȥCl2�е��Ȼ����ȥ�����е�ˮ�������ն��������ʹ�õĸ������ʯ��������Cl2�������ɫ��������ɫ��ʪ�����ɫ������ɫCl2��ˮ��Ӧ���ɴ����ᣬ���������Ư�����ü���HCl�����ӷ������ϡ���ٷ�����Ӧ��Ũ�����������¼���ʱ��С���������ȵ�MnO2��4H����2Cl��![]() Mn2����Cl2����2H2ONaOH��ҺCl2��2OH��=Cl����ClO����H2O
Mn2����Cl2����2H2ONaOH��ҺCl2��2OH��=Cl����ClO����H2O
��������
��1��ʵ������ȡ������ҪŨ���ᣬ���ڼ��ȵ������½��С����ɵ������к���ˮ�������Ȼ��⣬Ӧ���ȳ��Ȼ��⣬���ˮ��������Ӧ���dz��ڽ����̿ڳ������Դ����Тٲ�����ϡ���ᣬ��Ӧ��Ũ�����Ӧ�м���װ�ã�Ӧ�þƾ��Ƽ��ȡ���ʢŨ�����ʢ����ʳ��ˮ��ϴ��ƿλ�õߵ�������ϴ��ƿ��������ܺͳ������ܵij��̲��ԡ���2�����Ȼ��⼫������ˮ�����Ա���ʳ��ˮ�������dz�ȥCl2�е��Ȼ��⡣��Ũ���������ˮ�ԣ����Ũ����������dz�ȥ�����е�ˮ�������������ж�����Ҫβ��������������������Һ�����������ն����������
��3������Ϊ������һ���������壬�ܱ���ʯ�����գ����Եò���Ԥ���е�ʪ�����ɫ������ɫ������ʵ��ʧ�ܵ�ԭ����ʹ�õĸ������ʯ�ҽ�Cl2�����ˣ��ʴ�Ϊ��ʹ�õĸ������ʯ�ҽ�Cl2���գ�
�ھ���Ư���Ե��Ǵ����ᣬ���������������������ʺϵĸ����������ͨ����ɫ�����ϵ��Ǹ���������������ڸ������ɫ�����ϲ������ɴ����ᣬ���Ը������ɫ��������ɫ������ʪ�����ɫ�����Ͽ������ɾ���Ư���ԵĴ����ᣬ����ʪ�����ɫ������ɫ���ɴ˵ó�Cl2ʹ��ɫ������ɫ�Ļ�����Cl2��ˮ��Ӧ���ɴ����ᣬ���������Ư�����òŵ�����ɫ������ɫ���ʴ�Ϊ���������ɫ��������ɫ��ʪ�����ɫ������ɫ�� Cl2��ˮ��Ӧ���ɴ����ᣬ���������Ư�����ã�
��4��Ũ����Ͷ����������ڼ��ȵ������·�Ӧ��Ũ������лӷ��ԣ����Լ���ʹ����Ũ����ӷ���Ũ����Ͷ��������ܷ���������ԭ��Ӧ�����Ȼ��̡�������ˮ���淴Ӧ���У�Ũ����Ũ�ȼ�С��ϡ���ܺͶ������̷�Ӧ������������������������������0.06mol���ʴ�Ϊ���ټ���ʹ����Ũ����ӷ���������Ũ�ȱ�ϡ���ٷ�����Ӧ��
��5����ԣ�4���з����������ٵ�ԭ�ɲ�ȡ��Ũ�����������룬��С����ȵȴ�ʩ�����Ũ����������ʣ�
��6��Բ����ƿ�����ö������̺�Ũ���Ṳ����ȡ������������Ӧ�����ӷ���ʽMnO2��4H����2Cl��![]() Mn2����Cl2����2H2O��ʵ�������ϴ����ʱ��Ϊ�˼�����ƿ�в��������Ի�������Ⱦ��ѡ�ü�Һ��NaOH��Һ�����ն�����������йص����ӷ���ʽ��Cl2��2OH��=Cl����ClO����H2O��
Mn2����Cl2����2H2O��ʵ�������ϴ����ʱ��Ϊ�˼�����ƿ�в��������Ի�������Ⱦ��ѡ�ü�Һ��NaOH��Һ�����ն�����������йص����ӷ���ʽ��Cl2��2OH��=Cl����ClO����H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�