��Ŀ����
A��B��C��D��E����ѧ������5�ֻ����A��B�������X��Y�������г����Ľ������ʣ�������ʼ�Ĺ�ϵ����ͼ��ʾ��
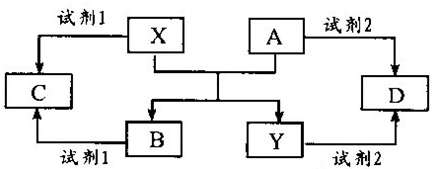
��ش��������⣺
��1��д��X��A��Ӧ�Ļ�ѧ����ʽ��______��
��2�����Լ�1��NaOH��Һ��д��X���Լ�1��Ӧ�����ӷ���ʽ______��
��3�����Լ�1���Լ�2����ϡ���ᣮ
�ټ�������D����Һ�н������ӵķ�����______��
�ڽ�����C����ˮ������Һ�����ԣ�ԭ����______ �������ӷ���ʽ��ʾ����
��ij��Ч��ˮ������Y��OH��SO4�ۺϵõ��ģ���ҵ����D��ϡ�������������Ϊԭ�����Ʊ�Y��OH��SO4����Ӧ����NO���ɣ��÷�Ӧ�Ļ�ѧ����ʽ��______��
��4�����Լ�1���Լ�2����ϡ���ᣬ��C��D��Ϻ�ͨ������Z���ٵμ�����������Һ������Һ��pH����ˮ��ۺϵõ���Ч��ˮ���ۺ��Ȼ�����[AlFe��OH��nCl6-n]m������Z��______����������______��
�⣺�⣺Ԫ��X��Y�ĵ����������г����Ľ�����Y�����������XΪAl��YΪFe��A��B���������Al����������Ӧ����Fe����������Al�����������������ᷴӦ������Ӧ����Fe���������������ᷴӦ�����Լ�1ΪNaOH��Һʱ��CΪƫ�����ƣ��Լ�2Ϊ����ʱ��DΪ�����������Լ�1Ϊ����ʱ��CΪ������������Һˮ�������ԣ�
��1��X�ĵ�����A��ӦΪ���ȷ�Ӧ����Al����������Ӧ������������Fe���÷�ӦΪ3FeO+2Al Al2O3+3Fe��
Al2O3+3Fe��
�ʴ�Ϊ��3FeO+2Al Al2O3+3Fe��
Al2O3+3Fe��
��2�����Լ�1��NaOH��Һ����Al��NaOH��Һ��Ӧ����ƫ�����ƺ����������ӷ�ӦΪ2Al+2H2O+2OH-�T2AlO2-+3H2�����ʴ�Ϊ��2Al+2H2O+2OH-�T2AlO2-+3H2����
��3�����Լ�1���Լ�2����ϡ���ᣬ
��DΪ�����������������ӵķ���Ϊȡ������Һ���Թ��У��μӼ���KSCN��Һ����ɫ���μ���ˮ����Һ���ɫ����֤��ԭ��Һ�к���Fe2+��
�ʴ�Ϊ��ȡ������Һ���Թ��У��μӼ���KSCN��Һ����ɫ���μ���ˮ����Һ���ɫ����֤��ԭ��Һ�к���Fe2+��
��CΪ��������ˮ�������ԣ�ˮ�����ӷ�ӦΪAl3++3H2O Al��OH��3+3H+��
Al��OH��3+3H+��
�ʴ�Ϊ��Al3++3H2O Al��OH��3+3H+��
Al��OH��3+3H+��
��EΪ������������ϡ�������������Ϊԭ������Fe��OH��SO4����Ӧ����NO���ɣ��÷�ӦΪ2FeSO4+2NaNO2+H2SO4�T2Fe��OH��SO4+Na2SO4+2NO����
�ʴ�Ϊ��2FeSO4+2NaNO2+H2SO4�T2Fe��OH��SO4+Na2SO4+2NO����
��4��CΪƫ�����ƣ�DΪ����������C��D��Ϻ�ͨ������Z������[AlFe��OH��nCl6-n]m����Ԫ���غ��֪��ZΪ�������ɽ�������������Ϊ�����ӣ�
�ʴ�Ϊ����������������������Ϊ�����ӣ�
������Ԫ��X��Y�ĵ����������г����Ľ�����Y�����������XΪAl��YΪFe��A��B���������Al����������Ӧ����Fe����������Al�����������������ᷴӦ������Ӧ����Fe�����������������ᷴӦ�����Լ�1ΪNaOH��Һʱ��CΪƫ�����ƣ��Լ�2Ϊ����ʱ��DΪ�����������Լ�1Ϊ����ʱ��CΪ������������Һˮ�������ԣ�����ϻ�ѧ��������ɣ�
���������⿼��������ƶϣ���ȷ���ȷ�Ӧ��Al����������Fe���������������ʼ��ɽ�𣬲�ע���Լ�1����Ϊ���ǿ�������������Ŀ�ѶȲ���
��1��X�ĵ�����A��ӦΪ���ȷ�Ӧ����Al����������Ӧ������������Fe���÷�ӦΪ3FeO+2Al
 Al2O3+3Fe��
Al2O3+3Fe���ʴ�Ϊ��3FeO+2Al
 Al2O3+3Fe��
Al2O3+3Fe����2�����Լ�1��NaOH��Һ����Al��NaOH��Һ��Ӧ����ƫ�����ƺ����������ӷ�ӦΪ2Al+2H2O+2OH-�T2AlO2-+3H2�����ʴ�Ϊ��2Al+2H2O+2OH-�T2AlO2-+3H2����
��3�����Լ�1���Լ�2����ϡ���ᣬ
��DΪ�����������������ӵķ���Ϊȡ������Һ���Թ��У��μӼ���KSCN��Һ����ɫ���μ���ˮ����Һ���ɫ����֤��ԭ��Һ�к���Fe2+��
�ʴ�Ϊ��ȡ������Һ���Թ��У��μӼ���KSCN��Һ����ɫ���μ���ˮ����Һ���ɫ����֤��ԭ��Һ�к���Fe2+��
��CΪ��������ˮ�������ԣ�ˮ�����ӷ�ӦΪAl3++3H2O
 Al��OH��3+3H+��
Al��OH��3+3H+���ʴ�Ϊ��Al3++3H2O
 Al��OH��3+3H+��
Al��OH��3+3H+����EΪ������������ϡ�������������Ϊԭ������Fe��OH��SO4����Ӧ����NO���ɣ��÷�ӦΪ2FeSO4+2NaNO2+H2SO4�T2Fe��OH��SO4+Na2SO4+2NO����
�ʴ�Ϊ��2FeSO4+2NaNO2+H2SO4�T2Fe��OH��SO4+Na2SO4+2NO����
��4��CΪƫ�����ƣ�DΪ����������C��D��Ϻ�ͨ������Z������[AlFe��OH��nCl6-n]m����Ԫ���غ��֪��ZΪ�������ɽ�������������Ϊ�����ӣ�
�ʴ�Ϊ����������������������Ϊ�����ӣ�
������Ԫ��X��Y�ĵ����������г����Ľ�����Y�����������XΪAl��YΪFe��A��B���������Al����������Ӧ����Fe����������Al�����������������ᷴӦ������Ӧ����Fe�����������������ᷴӦ�����Լ�1ΪNaOH��Һʱ��CΪƫ�����ƣ��Լ�2Ϊ����ʱ��DΪ�����������Լ�1Ϊ����ʱ��CΪ������������Һˮ�������ԣ�����ϻ�ѧ��������ɣ�
���������⿼��������ƶϣ���ȷ���ȷ�Ӧ��Al����������Fe���������������ʼ��ɽ�𣬲�ע���Լ�1����Ϊ���ǿ�������������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
�����Ŀ
��֪A��B��C��D��E�Ƕ�����ԭ�������������������Ԫ�أ�Aԭ����Ԫ�����ڱ���ԭ�Ӱ뾶��С��B��Eͬ���壬��E��ԭ��������B��������C��D�ǽ��������ǵ����������������ˮ������˵������ȷ���ǣ�������
| A�������ӵİ뾶��C��D��E��B | B����ҵ�ϳ��õ�ⷨ�Ƶ�C��D�ĵ��� | C���ȶ��ԣ�A2B��A2E | D������D������ұ��ijЩ���۽��� |

 2DB3����3.2gDB2��ȫת��ΪDB3����ʱ����akJ��1mol DB3������ȫת��Ϊ��ˮ�������bkJ��������33.6L DB2��ȫ�������������Ӧ����
2DB3����3.2gDB2��ȫת��ΪDB3����ʱ����akJ��1mol DB3������ȫת��Ϊ��ˮ�������bkJ��������33.6L DB2��ȫ�������������Ӧ����
