��Ŀ����
����Ŀ��A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ��������������A��B����ͬһ���ڣ�C��D��Eͬ����һ���ڡ�C��B�ɰ�ԭ�Ӹ�����2:1��1:1�ֱ��γ��������ӻ�������ҡ�D��A��ԭ�Ӹ�����3:2�γ����ӻ��������E�ǵؿ��к�����ߵĽ���Ԫ�ء�����������Ϣ�ش���������:
��1��BԪ�������ڱ��е�λ����_____________�������ʵĵ���ʽ��______________________��
��2��A��B��C��D��E����Ԫ�ص�ԭ�Ӱ뾶��С�����˳����____________(��Ԫ�ط�����д)
��3��E�ĵ�����C������������Ӧ��ˮ�������Һ������Ӧ�����ӷ���ʽ��______________________________________��
��4��Ԫ��A��ԭ�Ӻ����_____����״��ͬ�ĵ����ơ�
��5������Ԫ��C���ε���ɫ��ӦΪ________________ɫ����������ζ����Է�����ɫ��Ӧ����ԭ����________________________________________________________��
���𰸡� ������ VIA�� ![]() Na>Mg>Al>N>O 2Al+2OH-+2H2O=2AlO2-+3H2�� 2 �� ����̬�ĵ��Ӵ������ߵĹ��ԾǨ�������͵Ĺ��ʱ,��һ���IJ���(�ɼ�������)�����ʽ�ͷ�����
Na>Mg>Al>N>O 2Al+2OH-+2H2O=2AlO2-+3H2�� 2 �� ����̬�ĵ��Ӵ������ߵĹ��ԾǨ�������͵Ĺ��ʱ,��һ���IJ���(�ɼ�������)�����ʽ�ͷ�����
�����������������A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ��������������A��B����ͬһ���ڣ�C��D��Eͬ����һ���ڡ�C��B�ɰ�ԭ�Ӹ�����2:1��1:1�ֱ��γ��������ӻ������������BΪNa��CΪO����Ϊ�����ơ���Ϊ����������D��A��ԭ�Ӹ�����3:2�γ����ӻ����������AΪN��DΪMg����Ϊ��������þ��E�ǵؿ��к�����ߵĽ���Ԫ������EΪAl��
��1��BԪ�������ڱ��е�λ���Ƕ����� VIA�壬�����ʵĵ���ʽ��![]() ��
��
��2��A��B��C��D��E����Ԫ�ص�ԭ�Ӱ뾶��С�����˳����Na>Mg>Al>N>O ��
��3��E�ĵ�����C������������Ӧ��ˮ�������Һ������Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=2AlO2-+3H2����
��4��Ԫ��A�Ļ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p3��s��������εġ�p����ǷĴ��εģ�������ԭ�Ӻ����2����״��ͬ�ĵ����ơ�
��5������Ԫ��C���ε���ɫ��ӦΪ��ɫ����������ζ����Է�����ɫ��Ӧ����ԭ����������̬�ĵ��Ӵ������ߵĹ��ԾǨ�������͵Ĺ��ʱ,��һ���IJ���(�ɼ�������)�Ĺ����ʽ�ͷ�������

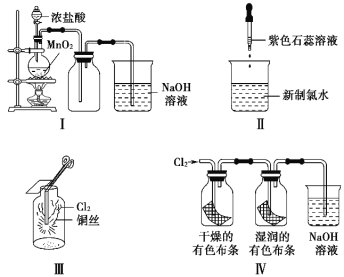
����Ŀ��ʵ��������ͼ��ʾװ���Ʊ�����������һϵ�����ʵ��(�г��豸����Ҫ�ļ���װ����ʡ��)��

(1)����װ��װ����Ϻ����Ƚ��еIJ�����______________________���������Լ���
(2)ʵ���ҳ���MnO2+4HCl(Ũ)![]() MnCl2+Cl2��+2H2O��ȡ������
MnCl2+Cl2��+2H2O��ȡ������
��ʵ��ʱ��ͨ�������ܶ�Ϊ1��19g/cm3��Ũ��Ϊ36��5%��Ũ���ᡣ��Ũ��������ʵ���Ũ��Ϊ___________________________��
�ڱ�״���£�������Ӧÿ����4.48L������ת�Ƶ��ӵ����ʵ���Ϊ_________ mol��
(3)ϴ��װ��B�������dz�ȥCl2�е�HCl���壬ͬʱ������ȫƿ(���ʵ��ʱװ��C���Ƿ�������)����װ��C����������װ��B�н��۲쵽��������_____________��
(4)װ��C����������֤�����Ƿ����Ư���ԡ�Ϊ�ˣ�ʵ��ʱװ��C��I��II��III�����η����������___________(����ĸ)��
A | B | C | D | |
I | �������ɫ���� | ʪ�����ɫ���� | �������ɫ���� | ʪ�����ɫ���� |
II | ��ʯ�� | ��ˮ�Ȼ��� | ��ˮ�Ȼ��� | Ũ���� |
III | ʪ�����ɫ���� | �������ɫ���� | ʪ�����ɫ���� | �������ɫ���� |
(5)���װ��D��E��Ŀ���DZȽ��ȡ��塢��������ԡ�����D�л���ͨ��һ��������ʱ�����Կ�����ɫ��Һ��Ϊ��ɫ��˵�������������Ա��嵥�ʵ�______(����ǿ������������)����������װ��D��������Һ����װ��E�У����ã��۲쵽��������___________��
(6)FΪβ������װ�á�ʵ��ʱ����װ���з�Ӧ�����ӷ���ʽ______________��