��Ŀ����
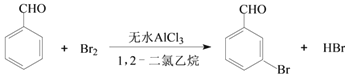
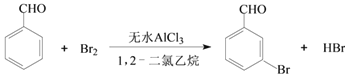
����Ŀ��ʵ�����Ա���ȩΪԭ���Ʊ����屽��ȩ�ķ�Ӧ���£�
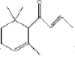
��֪��(1)���屽��ȩ�¶ȹ���ʱ�ױ�������
(2)�塢����ȩ��1,2-�������顢���屽��ȩ�ķе㼰��Է����������±���
���� | �� | ����ȩ | 1,2-�������� | ���屽��ȩ |
�е�/�� | 58.8 | 179 | 83.5 | 229 |
��Է������� | 160 | 106 | 185 |
����1����һ����ȵ���ˮAlCl3��1,2-��������ͱ���ȩ��ֻ�Ϻ�װ��������ƿ(��ͼ��ʾ)�������μӾ�Ũ��������������Һ�壬���·�Ӧһ��ʱ�䣬��ȴ��
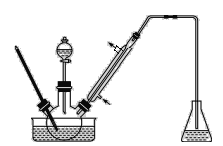
����2������Ӧ����ﻺ������һ������ϡ�����У����衢���á���Һ���л�����10% NaHCO3��Һϴ�ӡ�
����3����ϴ�ӵ��л������������ˮMgSO4���壬����һ��ʱ�����˳�MgSO4nH2O���塣
����4����ѹ�����л��㣬�ռ���Ӧ��֡�
(1)ʵ��װ���������ܵ���Ҫ������_________����ƿ��ӦΪ_____(�ѧʽ)��Һ��
(2)����1��Ӧ�����У�Ϊ���ԭ�������ʣ����˵��¶ȷ�ΧΪ(�����)_______��
A����229�� B��58.8��~179�� C����58.8��
(3)����2����10% NaHCO3��Һϴ�ӣ���Ϊ�˳�ȥ�����л����_______(�ѧʽ)��
(4)����3�м�����ˮMgSO4�����������_____________��
(5)����4�в��ü�ѹ������Ϊ�˷�ֹ__________________________________��
(6)��ʵ���м�����5.3 g����ȩ���õ�3.7 g���屽��ȩ������屽��ȩ����Ϊ______��
���𰸡��������� NaOH C Br2 ��ȥ�л����ˮ ���屽��ȩ���¶ȹ��߱����� 40%
��������
��1�������ܵ�������:��������,�����ԭ�ϵ�������,��ƿ��ʢ�ŵ���NaOH��Һ�����շе�ϵ͵���;�����Ϊ:����������NaOH ��
(2)����1��Ӧ������,Ϊ�����ԭ��������,Ӧ�����¶ȵ�����ͱ���ȩ�ķе�,�����¶�Ӧ����58.8��C;�����Ϊ:C��
(3)����2�л����е�������NaHCO3��Һ��Ӧ,�����л�����10%NaHCO3��Һϴ�ӣ���Ϊ�˳�ȥ��;�����Ϊ:Br2��
��4����ϴ�ӵ��л������������ˮMgSO4����,����-��ʱ�����˳�MgSO4![]() nH2O����,���Բ���3�м�����ˮMgSO4����,��Ϊ�˳�ȥ�л����е�ˮ;�����Ϊ:��ȥ�л����ˮ��
nH2O����,���Բ���3�м�����ˮMgSO4����,��Ϊ�˳�ȥ�л����е�ˮ;�����Ϊ:��ȥ�л����ˮ��
(5)���ü�ѹ����,���Խ��������ķе�,��˿����ڽϵ͵��¶��·�������,��ֹ���屽��ȩ���¶ȹ��߱�����;�����Ϊ:���屽��ȩ���¶ȹ��߱�������
(6)���� ����5.3g����ȩ��ȫ��Ӧ����xg���屽��ȩ������106:185=5.3:x,���x=9.25g,���Լ��屽��ȩ����3.7g/9.25g
����5.3g����ȩ��ȫ��Ӧ����xg���屽��ȩ������106:185=5.3:x,���x=9.25g,���Լ��屽��ȩ����3.7g/9.25g ![]() 100%=40%;�����Ϊ: 40%��
100%=40%;�����Ϊ: 40%��

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�����Ŀ����֪25 ��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ,�ش���������:
��ѧʽ | CH3COOH | H2CO3 | HClO |
����ƽ�ⳣ�� | Ka=1.8��10-5 |
| Ka=3.0��10-8 |
��1�����ʵ���Ũ�Ⱦ�Ϊ0.1 mol��L-1������������Һ,pH��С�������е�˳����__________(�ñ����д)��
a.CH3COONa b.Na2CO3 c.NaClO d.NaHCO3
��2��������,0.1 mol��L-1 CH3COOH��Һ��ˮϡ������,���б���ʽ�����ݱ�����__________(����ĸ)��
a. ![]() b.
b. ![]() c.
c. ![]()
d. ![]() e.
e. ![]()
��3��д�������������Һ��ͨ������������̼�����ӷ���ʽ__________��
��4��25��ʱ,CH3COOH��CH3COONa�Ļ����Һ,����û��ҺpH=6,����Һ��:c(CH3COO-)-c(Na+)=__________(��ȷ��ֵ)��
��5��25��ʱ,��a mol��L-1�Ĵ�����b mol��L-1�������Ƶ�������,��Ӧ����Һǡ��������,��a��b��ʾ����ĵ��볣��Ϊ__________
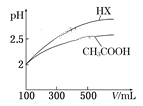
��6�������Ϊ100 mL pH=2��CH3COOH��һԪ��HX,��ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ,��HX�ĵ���ƽ�ⳣ��__________(����>������=������<��)CH3COOH�ĵ���ƽ�ⳣ����