��Ŀ����
����Ŀ����1�������£���A��B��C��D������ɫ��Һ�����Ƿֱ���CH3COONa��Һ��NH4Cl��Һ�������NaNO3��Һ�е�һ�֡���֪A��B��ˮ��Һ��ˮ�ĵ���̶���ͬ��A��C��Һ��pH��ͬ����B��________��C��________��
��2��ʵ��������FeSO4��Һ���ܽ�ʱ��Ҫ����������ϡ���ᣬ��ԭ����__________________________��������Ϻ�Ҫ����������м����Ŀ����__________________________����AlCl3��Һ�������գ����õ�����Ҫ���������___________��AlCl3��Һ�����Ե�ԭ���ǣ������ӷ���ʽ˵����__________________��
���𰸡� CH3COONa ���� Ϊ������Fe2+��ˮ�� ��ֹFe2+�������е�����������Fe3+ Al2O3 Al3++3H2O![]() Al(OH)3+3H+
Al(OH)3+3H+
�������������������1��CH3COONa��ǿ�������Σ�ˮ��ʼ��ԣ�NH4Cl��ǿ�������Σ�ˮ������ԣ�������Һ�����ԣ�NaNO3��ǿ��ǿ��������Һ�����ԣ���2������������Һ�У�������������Һ�з���ˮ�����������������������ӣ������������ױ������������ӣ�Al3+����ˮ�⣬ˮ��ķ���ʽΪAl3++3H2O![]() Al(OH)3+3H+��ˮ�����Һ�����ԣ����ɺ����չ����У�HCl�ӷ���Al(OH)3���ȶ�������ʱ�ֽ�����Al2O3��
Al(OH)3+3H+��ˮ�����Һ�����ԣ����ɺ����չ����У�HCl�ӷ���Al(OH)3���ȶ�������ʱ�ֽ�����Al2O3��
��������1��CH3COONa��Һ��NH4Cl��Һ�������NaNO3������Һ��CH3COONaΪǿ�������Σ�ˮ��ʼ��ԣ��ٽ�ˮ�ĵ��룬NH4Cl��ҺΪǿ�������Σ�ˮ������ԣ��ٽ�ˮ�ĵ��룬������Һ�����ԣ�����ˮ�ĵ��룬NaNO3Ϊǿ��ǿ���Σ���Һ�����ԣ�A��C��Һ��pH��ͬ��A��CΪNH4Cl��Һ�����A��B��Һ��ˮ�ĵ���̶���ͬ��A��BΪCH3COONa��Һ��NH4Cl��Һ����AΪNH4Cl��Һ��BΪCH3COONa��Һ��CΪ���ᣬDΪNaNO3��Һ��
��2��ʵ�������Ƶ�FeSO4��Һʱ�������������Ӳ���ˮ��������������������Ӧ�����ӷ���ʽΪ��Fe2++2H2O![]() Fe��OH��2+2H+��ͨ������ϡ���ᣬʹ������Ũ������ˮ��ƽ�������ƶ���������Fe2+ˮ�⣻����Fe2+���ױ�O2��������Ϊ��ɫ��Fe3+������������Ϻ�Ҫ����������м����ֹFe2+�������е�����������Fe3+���Ȼ���Ϊǿ�������Σ�Al3+����ˮ�⣬ˮ��ķ���ʽΪAl3++3H2O
Fe��OH��2+2H+��ͨ������ϡ���ᣬʹ������Ũ������ˮ��ƽ�������ƶ���������Fe2+ˮ�⣻����Fe2+���ױ�O2��������Ϊ��ɫ��Fe3+������������Ϻ�Ҫ����������м����ֹFe2+�������е�����������Fe3+���Ȼ���Ϊǿ�������Σ�Al3+����ˮ�⣬ˮ��ķ���ʽΪAl3++3H2O![]() Al(OH)3+3H+��ˮ�����Һ�����ԣ����ɺ����չ����У�HCl�ӷ���ˮ��ƽ�ⲻ�������ƶ�����ȫ������������������Al(OH)3���ȶ�������ʱ�ֽ�����Al2O3��
Al(OH)3+3H+��ˮ�����Һ�����ԣ����ɺ����չ����У�HCl�ӷ���ˮ��ƽ�ⲻ�������ƶ�����ȫ������������������Al(OH)3���ȶ�������ʱ�ֽ�����Al2O3��

����Ŀ����ͼ�Ǵ���ʿ����Ҫ�ɷ�ΪAl2O3 �� ����������SiO2��Fe2O3�����ʣ�����ȡAl2O3������AlN�Ĺ������̣� 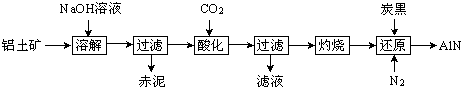
��1�����ܽ⡱ʱ����Һ�еĹ�������ƫ�����Ʒ�����Ӧ��2Na2SiO3+2NaAlO2+2H2O�TNa2Al2Si2O8��+4NaOH �������Ҫ�ɷ�Ϊ��д����ѧʽ����
��2�����ữ��ʱͨ�����CO2��NaAlO2��Ӧ����Al��OH��3 �� ��Һ����Ҫ�ɷ�Ϊ��д����ѧʽ����ʵ���ҹ������õ��IJ����������ձ�������������
��3������ԭ��ʱ��̿���ڸ����±�����ΪCO����Ӧ�Ļ�ѧ����ʽΪ ��
��4����ȡ���ݲ�ͬ�����ĵ�������Ʒ����������ֻ����̿�ڣ��ֱ�ӵ�20.00mL��ͬŨ�ȵ�NaOH��Һ�У���ַ�Ӧ���ʵ�����������ʾ�� ����֪��AlN+NaOH+H2O�TNaAlO2+NH3����
ʵ����� | �� | �� | �� |
���뵪������Ʒ������/g | 4.1 | 8.2 | 12.3 |
���ɰ��������/L����״���� | 1.456 | 2.912 | 4.256 |
�ٸ���Ʒ��AlN����������Ϊ���٣���д��������̣�