��Ŀ����
7���ش�����������1����25�������½�pH=11�İ�ˮϡ��100������Һ��pHΪ������ţ�D��
A��9 B��13 C��11��13֮�� D��9��11֮��
��2��25��ʱ����0.1mol/L�İ�ˮ�м��������Ȼ�粒��壬�������ܽ�����ҺpH��С����Ҫԭ���ǣ�����ţ�C��
A����ˮ���Ȼ�立�����ѧ��Ӧ
B���Ȼ����Һˮ�������ԣ�������c��H+��
C���Ȼ������ˮ���������������ӣ������˰�ˮ�ĵ��룬ʹc��OH-����С
��3�������£�����0.1mol NH4Cl��0.05mol NaOHȫ������ˮ���γɻ����Һ����������ʧ����
��NH3•H2O��NH4+�������ӵ����ʵ���֮�͵���0.1mol��
��NH4+��H+�������ӵ����ʵ���֮�ͱ�OH-��0.05mol��
��4����֪ij��Һ��ֻ����OH-��H+��NH4+��Cl-�������ӣ�ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ
A��c��Cl-����c��NH4+����c��H+����c��OH-�� B��c��Cl-����c��NH4+����c��OH-����c��H+��
C��c��Cl-����c��H+����c��NH4+����c��OH-�� D��c��NH4+����c��Cl-����c��OH-����c��H+��
������Һ��ֻ�ܽ���һ�����ʣ������ʵ��������Ȼ�泥���������Ũ�ȴ�С˳���ϵ����ȷ���ǣ�ѡ����ţ�A��
��������Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ǡ�ó����ԣ�
����ǰc��HCl���������������������=������ͬ���� c��NH3•H2O����
��Ϻ���Һ��c��NH4+����c��Cl-���Ĺ�ϵc��NH4+��= c��Cl-����
���� ��1��һˮ�ϰ���������ʣ���ˮ�ĵ��뷽��ʽΪ��NH3•H2O?NH4++OH-����ˮϡ�ʹٽ���ˮ�ĵ��룬��pH=11�İ�ˮϡ��100����ϡ�ͺ����Һ������������Ũ�ȴ���ԭ����$\frac{1}{100}$��
��2����ˮ��������ʣ����ڵ���ƽ�⣬����Һ�м�����ͬ�����������ư�ˮ���룬�ݴ˻ش��жϣ�
��3������0.1mol NH4Cl��0.05mol NaOHȫ������ˮ�γɻ����Һ����Һ�д���NH4+��NH3•H2O����������غ�͵���غ���
��4��A����Һ�����ԣ�����ΪNH4Cl��Һ��NH4Cl��HCl�Ļ���
B��������Ũ�ȴ���������Ũ�ȣ������ܴ������������
C����Һ�����ԣ���c��H+����c��NH4+����ӦΪNH4Cl��HCl�Ļ���
D����Һ�ʼ��ԣ���c��NH4+����c��Cl-����ӦΪNH3•H2O��NH4Cl�Ļ���
��� �⣺��1��һˮ�ϰ�Ϊ������ʣ����ڵ���ƽ�⣬ϡ�ͺ�һˮ�ϰ��ĵ���̶�������Һ�����������ӵ����ʵ����������Խ�pH=11�İ�ˮϡ��100����ϡ�ͺ����Һ������������Ũ�ȴ���ԭ����$\frac{1}{100}$����Һ��pHӦ��9-11֮�䣬
��ѡD��
��2��һˮ�ϰ���������ʣ���Һ�д��ڵ���ƽ�⣬����Һ�м����Ȼ�泥�笠�����Ũ����������һˮ�ϰ����룬������Һ������������Ũ�ȼ�С����Һ��pH��С��
A����ˮ���Ȼ�鱗�������ѧ��Ӧ����A����
B���Ȼ����Һˮ�������ԣ���笠�����Ũ��ԶԶ����������Ũ�ȣ�����笠���������һˮ�ϰ�����Ϊ����������Ũ�ȼ�С����B����
C���Ȼ������ˮ�����������笠����ӣ������˰�ˮ�ĵ��룬ʹc��OH-����С����C��ȷ��
��ѡ��C��
��3���ٸ���Nԭ���غ��֪����Һ��NH3•H2O��NH4+�������ӵ����ʵ���֮�͵���0.1mol���ʴ�Ϊ��NH3•H2O��NH4+��
�ڸ��ݵ���غ�ʽc��NH4+��+c��H+��+c��Na+��=c��OH-��+c��Cl-������c��NH4+��+c��H+��-c��OH-��=c��Cl-��-c��Na+��=0.1mol-0.05mol���ʴ�Ϊ��NH4+��H+��
��4������Һ��ֻ����OH-��H+��NH4+��Cl-�������ӣ�����ΪNH4Cl��Һ����NH4+ˮ��������ԣ���Һ������Ũ�ȴ�С˳��Ϊc��Cl-����c��NH4+����c��H+����c��OH-�����ʴ�Ϊ��NH4Cl��A��
�ڣ���Һ�����ԣ���c��Cl-��+c��OH-��=c��NH4+��+c��H+���ɵ�c��Cl-��=c��NH4+������ˮΪ������ʣ�������Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ˮŨ�ȴ�������Ũ�ȣ���С�ڻ���ڣ�����Һ�����ԣ�
�ʴ�Ϊ������=��
���� �����ۺϿ��������ˮ�⡢������ʵĵ����Լ�����Ũ�ȵĴ�С�Ƚϣ���Ŀ�ѶȽϴ�ע����������ˮ���Լ�������ʵ�������������ձȽ�����Ũ�ȴ�С˳��ķ���Ӱ�����ƽ���ƶ������أ�ѧ��ƽ���ƶ�ԭ����Ӧ���ǽ���Ĺؼ���

 ̼�������ǻ�����ѧ���о����ȵ���⣮
̼�������ǻ�����ѧ���о����ȵ���⣮I��ij�о�С���ֽ�����CO��g����H2O��g���Ļ������ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У�һ�������·�����Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g����H��0���õ�������ݣ�
| ʵ���� | �¶�/�� | ��ʼ����mol�� | ƽ������mol�� | �ﵽƽ���� ��Ҫʱ��/min | ||
| CO��g�� | H2O��g�� | CO2��g�� | H2��g�� | |||
| I | 800 | 2 | 2 | x | 1 | 5 |
| II | 900 | 1 | 2 | 0.5 | 0.5 | T1 |
| III | 900 | 2 | 2 | a | a | T2 |
��2���������ж���800��ʵ��������CO��g����H2O��g����Ӧһ���ﵽƽ��״̬����BD��
A��������ѹǿ���ٱ仯 B��n2��H2��=n��H2O��•n��CO��
C����������ܶȲ��� D��������CO��=������CO2��
��3��ʵ��II��III��CO��ƽ��ת���ʣ���II��CO������III��CO�� �����������=����ͬ����T1��T2��a=$\sqrt{3}$-1���ȷ��ֵ����
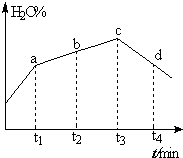
��4����ʵ����������Ϊ�ھ��ȵ��ܱ������н��У�ʵ����H2O��g����ת������ʱ��仯��ʾ��ͼ��ͼ��ʾ��b����������������������=����������t3��t4ʱ�̣�H2O��g����ת����H2O%���͵�ԭ���Ǹ÷�Ӧ�ﵽƽ�����ӦΪ���ȷ�Ӧ�ҷ�Ӧ����Ϊ�������������������¶����ߣ���Ӧ������У�
��5��CO��H2��һ�������ºϳɼ״����״�/��������ȼ�ϵ���У�����32g�״����������ת��4.5mol���ӣ������ĵ缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O���õ���е���Ч��Ϊ75%��������Ч�ʦ�=$\frac{ʵ��ת�Ƶ�����}{����ת�Ƶ�����}$��100%��
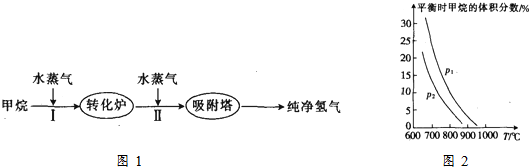
��1����I�����ķ�ӦΪCH4��g��+H2O��g��?CO��g��+3H2��g����
��д���÷�Ӧ��ƽ�ⳣ������ʽ$\frac{c��CO����{c}^{3}��{H}_{2}��}{c��C{H}_{4}����c��{H}_{2}O��}$��
����֪�ڡ�ˮ̼�ȡ�$\frac{c��{H}_{2}O��}{c��C{H}_{4}��}$=3ʱ������¶ȣ�T ����ѹǿ��p����������Ӧ��Ӱ����ͼ2��ʾ���������¶ȣ��÷�Ӧ��ƽ�ⳣ��K�������������С�����䡱������ͼ��֪P1��P2�������������������=����
��2�������ķ�ӦΪCO��g��+H2O��g��?CO2��g��+H2 ��g����T1�¶�ʱ����2L�ĺ����ܱ�������ͨ��һ������CO��H2O��g������Ӧ�����в�ò������������ʾ������t1��t2����
| ��Ӧʱ�䣨min�� | n��CO����mol�� | N��H2O����mol�� |
| 0 | 1.20 | 0.60 |
| t1 | 0.80 | |
| t2 | 0.20 |
�����ﵽƽ����������������䣬ֻ����ԭƽ����ϵ����ͨ��0.20mol H2O��g����������˵����ȷ����ab��
a��CO��ת���ʽ����� b��H2O��g�����������������
c��������ܶȽ����� d����ѧƽ�ⳣ��������
| A�� | ���Ȼ�����Һ�м������ϡ��ˮ��Al3++4OH-�TAlO2-+2H2O | |
| B�� | ��С�մ���Һ�м��������Һ��HCO3-+CH3COOH�TCH3COO-+CO2��+H2O | |
| C�� | ��������Һ��ͨ�������̼CO2+H2O+2C6H5O-��2C6H5OH+CO32- | |
| D�� | ���廯������Һ��ͨ���������Fe2++2Br-+2Cl2�TFe3++Br2+4Cl- |
CH4��g��+2O2��g���TCO2��g��+2H2O��l����H2
CH4��g��+$\frac{1}{2}$O2��g���TCH3OH��l����H3
H2O��g���TH2O��l����H4
CH3OH��l��+$\frac{3}{2}$O2��g���TCO2��g��+2H2O��l����H5
���й���������Ӧ�ʱ���жϲ���ȷ���ǣ�������
| A�� | ��H1����H2 | B�� | ��H2=��H1+��H4 | C�� | ��H3=��H2-��H5 | D�� | ��H4��0 |
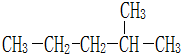 ��2-�����飻
��2-�����飻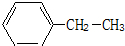 �ұ�
�ұ� 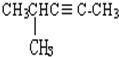 4-��-2-��Ȳ��
4-��-2-��Ȳ��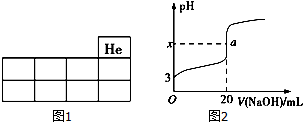 ij�̬������W��X��Y��Z 4�ֶ�����Ԫ����ɣ�����W��ԭ�Ӱ뾶��С��
ij�̬������W��X��Y��Z 4�ֶ�����Ԫ����ɣ�����W��ԭ�Ӱ뾶��С�� ��
�� ��
��