��Ŀ����
ijͬѧ�������ϲ鵽���·�Ӧ��

A��BΪ��ѧ��ѧ�������ʣ�AO2��BO2����ʹ����ʯ��ˮ����ǵ����壮
�ش��������⣺
��1����Ԫ��A��B���γɻ�����AB2����BԪ�ص�ԭ�ӽṹʾ��ͼΪ ��
������Ӧ��A��B��������Ϊ3��4����n��KClO3����n��AO2��= ��
����֪��A��s��+O2��g���TAO2��g������H=-393.5kJ?mol-1
B��s��+O2��g���TBO2��g������H=-296.8kJ?mol-1
A��s��+2B��s���TAB2��l������H=+89.7kJ?mol-1
д��AB2��l����O2����ȫȼ�յ��Ȼ�ѧ����ʽ ��
�ܻ�ɫ����ClO2��������ˮɱ��������ˮ������KClO3��BO2��ǿ������Һ�з�Ӧ���Ƶ�ClO2��д���˷�Ӧ�����ӷ���ʽ ��
��2����663K��303kPa�ʹ������ڵ������£�������Ӧ��AO+2H2?AH3OH������������ݣ�
��0��5min����ѧ��Ӧ����v��H2��= ��
����֪���¶��¸÷�Ӧ��ƽ�ⳣ��K=3����Ӧ���е�10minʱ ����ǡ����ﵽƽ��״̬���������� ��

A��BΪ��ѧ��ѧ�������ʣ�AO2��BO2����ʹ����ʯ��ˮ����ǵ����壮
�ش��������⣺
��1����Ԫ��A��B���γɻ�����AB2����BԪ�ص�ԭ�ӽṹʾ��ͼΪ
������Ӧ��A��B��������Ϊ3��4����n��KClO3����n��AO2��=
����֪��A��s��+O2��g���TAO2��g������H=-393.5kJ?mol-1
B��s��+O2��g���TBO2��g������H=-296.8kJ?mol-1
A��s��+2B��s���TAB2��l������H=+89.7kJ?mol-1
д��AB2��l����O2����ȫȼ�յ��Ȼ�ѧ����ʽ
�ܻ�ɫ����ClO2��������ˮɱ��������ˮ������KClO3��BO2��ǿ������Һ�з�Ӧ���Ƶ�ClO2��д���˷�Ӧ�����ӷ���ʽ
��2����663K��303kPa�ʹ������ڵ������£�������Ӧ��AO+2H2?AH3OH������������ݣ�
| t/min | 0 | 5 | 10 |
| AO/mol?L-1 | 1.00 | 0.65 | 0.50 |
| H2/mol?L-1 | 2.00 | 1.00 | |
| AH3OH/mol?L-1 | 0.00 | 0.35 | 0.50 |
����֪���¶��¸÷�Ӧ��ƽ�ⳣ��K=3����Ӧ���е�10minʱ
��������1����A��BΪ��ѧ��ѧ�������ʣ�AO2��BO2����ʹ����ʯ��ˮ����ǵ����壬BO2��ʹƷ����Һ��ɫ�������ƶ�BO2ΪSO2��AO2ΪCO2��ȷ��AΪC��BΪS��
�ڸ����غ���ƽ��ѧ����ʽ������ߵ����ʵ���֮�ȼ��ɣ�
�۸����Ȼ�ѧ����ʽ����д�����Լ���˹����������ش�
�ܸ�������������Ӧ��Ͳ������Ϣ����д��ѧ����ʽ��
��2���ٸ��ݷ���ʽϵ��֮�ȵ��ڱ仯��֮�ȼ��������ı仯Ũ�ȣ����������ʼ��㹫ʽ�����㼴�ɣ�
�ڸ���Ũ���ع����жϻ�ѧ��Ӧ�Ƿ�ﵽƽ��״̬��
�ڸ����غ���ƽ��ѧ����ʽ������ߵ����ʵ���֮�ȼ��ɣ�
�۸����Ȼ�ѧ����ʽ����д�����Լ���˹����������ش�
�ܸ�������������Ӧ��Ͳ������Ϣ����д��ѧ����ʽ��
��2���ٸ��ݷ���ʽϵ��֮�ȵ��ڱ仯��֮�ȼ��������ı仯Ũ�ȣ����������ʼ��㹫ʽ�����㼴�ɣ�
�ڸ���Ũ���ع����жϻ�ѧ��Ӧ�Ƿ�ﵽƽ��״̬��
����⣺A��B����ѧ��ѧ�������ʣ�AO2��BO2����ʹ����ʯ��ˮ����ǵ����壬BO2��ʹƷ����Һ��ɫ�������ƶ�BO2ΪSO2��AO2ΪCO2��ȷ��AΪC��BΪS��
��1����BԪ��ΪSԪ�أ�ԭ�ӽṹʾ��ͼΪ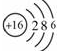 ��
��
�ʴ�Ϊ�� ��
��
������Ӧ��AΪC��BΪS��������Ϊ3��4�����ʵ���֮��=
��
=2��1��
����ԭ���غ���ƽ��ѧ����ʽ�õ���2KClO3+2C+S�T2KCl+2CO2+SO2��
�õ�n��KClO3����n��CO2��=1��1��
�ʴ�Ϊ��1��1��
�ۻ�ɫ����ClO2��������ˮɱ��������ˮ������KClO3��BO2��ǿ������Һ�з�Ӧ���Ƶ�ClO2������������ԭ��Ӧ�ĵ����غ��ԭ���غ�д�����ӷ���ʽΪ2ClO3-+SO2�T2ClO2+SO42-��
�ʴ�Ϊ��2ClO3-+SO2�T2ClO2+SO42-��
����֪��a��S��s��+O2��g���TSO2��g������H=-393.5kJ?mol-1
b��S��s��+O2��g���TSO2��g������H=-296.8kJ?mol-1
c��C��s��+2S��s���TCS2��l������H=+89.7kJ?mol-1
����̼ȼ�յķ�ӦCS2��l��+3O2��g���T2SO2��g��+CO2��g������H=a+2b-c�����ԡ�H=��-393.5kJ?mol-1��+2��-296.8kJ?mol-1��-89.7kJ?mol-1=-1076.8kJ?mol-1��
�ʴ�Ϊ��CS2��l��+3O2��g���T2SO2��g��+CO2��g������H=-1076.8kJ?mol-1��
��KClO3��SO2��ǿ������Һ�з�Ӧ�Ƶ�ClO2�����ӷ���ʽΪ��2ClO3-+SO2�T2ClO2+SO42-��
�ʴ�Ϊ��2ClO3-+SO2�T2ClO2+SO42-��
��2����0��5min�ڣ����ݱ仯��֮�ȵ���ϵ��֮�ȵó�5minʱ��������Ũ����1.30mol/L���仯Ũ����0.7mol/L����ѧ��Ӧ����v��H2��=
mol?L-1min-1=0.14mol?L-1min-1��
�ʴ�Ϊ��0.14mol?L-1min-1��
�ڵ���Ӧ���е�10minʱ��Qc=
=1��K�����Բ���ƽ��״̬��
�ʴ�Ϊ����ʱQ=1��K��
��1����BԪ��ΪSԪ�أ�ԭ�ӽṹʾ��ͼΪ
 ��
���ʴ�Ϊ��
 ��
��������Ӧ��AΪC��BΪS��������Ϊ3��4�����ʵ���֮��=
| 3 |
| 12 |
| 4 |
| 32 |
����ԭ���غ���ƽ��ѧ����ʽ�õ���2KClO3+2C+S�T2KCl+2CO2+SO2��
�õ�n��KClO3����n��CO2��=1��1��
�ʴ�Ϊ��1��1��
�ۻ�ɫ����ClO2��������ˮɱ��������ˮ������KClO3��BO2��ǿ������Һ�з�Ӧ���Ƶ�ClO2������������ԭ��Ӧ�ĵ����غ��ԭ���غ�д�����ӷ���ʽΪ2ClO3-+SO2�T2ClO2+SO42-��
�ʴ�Ϊ��2ClO3-+SO2�T2ClO2+SO42-��
����֪��a��S��s��+O2��g���TSO2��g������H=-393.5kJ?mol-1
b��S��s��+O2��g���TSO2��g������H=-296.8kJ?mol-1
c��C��s��+2S��s���TCS2��l������H=+89.7kJ?mol-1
����̼ȼ�յķ�ӦCS2��l��+3O2��g���T2SO2��g��+CO2��g������H=a+2b-c�����ԡ�H=��-393.5kJ?mol-1��+2��-296.8kJ?mol-1��-89.7kJ?mol-1=-1076.8kJ?mol-1��
�ʴ�Ϊ��CS2��l��+3O2��g���T2SO2��g��+CO2��g������H=-1076.8kJ?mol-1��
��KClO3��SO2��ǿ������Һ�з�Ӧ�Ƶ�ClO2�����ӷ���ʽΪ��2ClO3-+SO2�T2ClO2+SO42-��
�ʴ�Ϊ��2ClO3-+SO2�T2ClO2+SO42-��
��2����0��5min�ڣ����ݱ仯��֮�ȵ���ϵ��֮�ȵó�5minʱ��������Ũ����1.30mol/L���仯Ũ����0.7mol/L����ѧ��Ӧ����v��H2��=
| 0.7 |
| 5 |
�ʴ�Ϊ��0.14mol?L-1min-1��
�ڵ���Ӧ���е�10minʱ��Qc=
| 0.5 |
| 0.5��12 |
�ʴ�Ϊ����ʱQ=1��K��
������������һ���ۺ�֪ʶ�Ŀ����⣬����ѧ�����ɺ�����������ע��֪ʶ��Ǩ�ƺ�Ӧ���ǹؼ����Ѷȴ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 Cu��OH��2+2H+
Cu��OH��2+2H+
 4CO��g��+BaS��s����H1=+571.2kJ•mol-1
��
4CO��g��+BaS��s����H1=+571.2kJ•mol-1
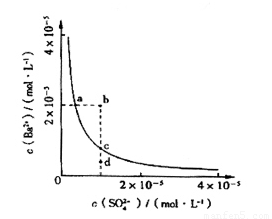
��
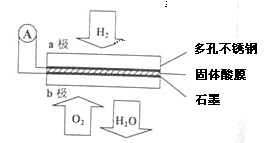
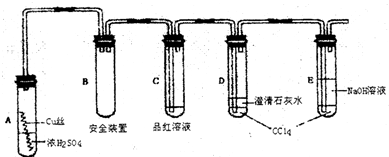
 Cu2S����ɫ��
Cu2S����ɫ��