��Ŀ����
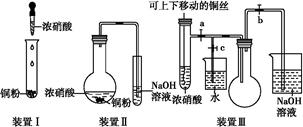
ͼ������ʵ���ҽ��а��������Ʊ�������ʵ������װ�ã����̶ֹ�װ��δ������
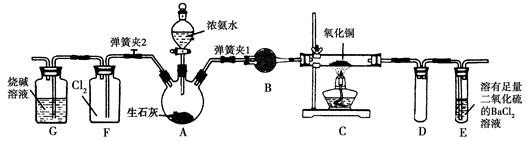
(1)����װ��װ�ú���Ҫ����A��Eװ�õ������ԣ������������________��Ȼ����A���۲쵽E��������ð�����ƿ��ƾ��ƻ��ɿ�˫�֣�E�е�����ˮ���γɣ�˵��װ�����������á�
(2)װ��B��ʢ�ŵ��Լ���________��
(3)��ȼC���ƾ��ƣ��رյ��ɼ�2�����ɼ�1���ӷ�Һ©���ų�Ũ��ˮ����û��ƿ�й����رշ�Һ©�����Ժ�Ƭ�̣�װ��C�к�ɫ������죬װ��E����Һ����ִ������ݣ�ͬʱ����________(������)����E���ݳ�Һ����������ֱ�������������д����C�з�����Ӧ�Ļ�ѧ����ʽ��________________________��
(4)��C�й���ȫ�����ɫ�رյ��ɼ�1�������ƿ��ƾ��ƣ�����ȴ����C�й�������������Ӧǰ��������Ϊ16 g����Ӧ����ع�����������2.4 g��ͨ������ȷ���ù������ijɷ���_________
__________(�û�ѧʽ��ʾ)��
(5)�ڹرյ��ɼ�1���ɼ�2�������������F�У��ܿ췢��װ��F�в������̣�ͬʱ����G����ҺѸ�ٵ�������F�У�д���������̵Ļ�ѧ����ʽ��____________________��Ѹ�ٲ���������ԭ����____________________________��

(1)����װ��װ�ú���Ҫ����A��Eװ�õ������ԣ������������________��Ȼ����A���۲쵽E��������ð�����ƿ��ƾ��ƻ��ɿ�˫�֣�E�е�����ˮ���γɣ�˵��װ�����������á�
(2)װ��B��ʢ�ŵ��Լ���________��
(3)��ȼC���ƾ��ƣ��رյ��ɼ�2�����ɼ�1���ӷ�Һ©���ų�Ũ��ˮ����û��ƿ�й����رշ�Һ©�����Ժ�Ƭ�̣�װ��C�к�ɫ������죬װ��E����Һ����ִ������ݣ�ͬʱ����________(������)����E���ݳ�Һ����������ֱ�������������д����C�з�����Ӧ�Ļ�ѧ����ʽ��________________________��
(4)��C�й���ȫ�����ɫ�رյ��ɼ�1�������ƿ��ƾ��ƣ�����ȴ����C�й�������������Ӧǰ��������Ϊ16 g����Ӧ����ع�����������2.4 g��ͨ������ȷ���ù������ijɷ���_________
__________(�û�ѧʽ��ʾ)��
(5)�ڹرյ��ɼ�1���ɼ�2�������������F�У��ܿ췢��װ��F�в������̣�ͬʱ����G����ҺѸ�ٵ�������F�У�д���������̵Ļ�ѧ����ʽ��____________________��Ѹ�ٲ���������ԭ����____________________________��
(1)�رյ��ɼ�2�ͷ�Һ©�����������ɼ�1����E��װ��ˮ
(2)��ʯ��(����ʯ��)
(3)��ɫ������2NH3��3CuO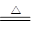 3Cu��N2����3H2O
3Cu��N2����3H2O
(4)Cu2O��Cu
(5)3Cl2��8NH3=N2��6NH4Cl
ʢ�������ļ���ƿ�����������������ɲ��ֹ��壬����ƿ��ѹǿ��С��������Һ����
(2)��ʯ��(����ʯ��)
(3)��ɫ������2NH3��3CuO
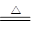 3Cu��N2����3H2O
3Cu��N2����3H2O(4)Cu2O��Cu
(5)3Cl2��8NH3=N2��6NH4Cl
ʢ�������ļ���ƿ�����������������ɲ��ֹ��壬����ƿ��ѹǿ��С��������Һ����
A���Ʊ�NH3��װ�ã�B�Ǹ���NH3��װ�ã�C��NH3��CuO�ķ�Ӧװ�ã�E�Ǵ���NH3��β��װ�ã�F��NH3��Cl2�ķ�Ӧװ�ã�G�Ǵ���β��Cl2��װ�á�
(2)����NH3��ʹ�ü��Ը������(3)C��CuO��NH3����������ԭ��Ӧ���ɺ�ɫ��Cu��N2��H2O��E�з�����Ӧ��2NH3��BaCl2��SO2��H2O=BaSO3����2NH4Cl��(4)������ȫ����Cu�����ɲ�����֪��С�IJ���ȫ����OԪ�ص���������n(CuO)��n(O)��2.4 g��16 g��mol��1��0.15 mol�����Է�Ӧ��CuO������Ϊ0.15 mol��80 g/mol��12 g<16 g������CuOδ��ȫת����Cu��ʣ������п��ܺ���Cu2O��(5)Cl2�ɽ�NH3����ΪN2��ͬʱ���ɵ�HCl���NH3��Ӧ����NH4Cl��������������Fƿ��ѹǿ��С�Ӷ����ֵ�������
�㲦�����⿼��NH3���Ʊ������ʡ���ѧʵ��������������鿼�������֪ʶ��Ӧ���������Ѷ��еȡ�
(2)����NH3��ʹ�ü��Ը������(3)C��CuO��NH3����������ԭ��Ӧ���ɺ�ɫ��Cu��N2��H2O��E�з�����Ӧ��2NH3��BaCl2��SO2��H2O=BaSO3����2NH4Cl��(4)������ȫ����Cu�����ɲ�����֪��С�IJ���ȫ����OԪ�ص���������n(CuO)��n(O)��2.4 g��16 g��mol��1��0.15 mol�����Է�Ӧ��CuO������Ϊ0.15 mol��80 g/mol��12 g<16 g������CuOδ��ȫת����Cu��ʣ������п��ܺ���Cu2O��(5)Cl2�ɽ�NH3����ΪN2��ͬʱ���ɵ�HCl���NH3��Ӧ����NH4Cl��������������Fƿ��ѹǿ��С�Ӷ����ֵ�������
�㲦�����⿼��NH3���Ʊ������ʡ���ѧʵ��������������鿼�������֪ʶ��Ӧ���������Ѷ��еȡ�

��ϰ��ϵ�д�
�����Ŀ


 3Cu+N2+3H2O������������þ�ڸ����·�Ӧ�ɵõ�����þ��������þ��ˮ������Ӧ����Mg��OH��2��NH3����������������Ʊ�����þ��ʵ�鷽��ʾ���ͼ��ʵ��ǰϵͳ�ڿ������ų���ͼ�м�ͷ��ʾ�������������Ϊ�˷����Ƿ���ȷ����˵������ ��
3Cu+N2+3H2O������������þ�ڸ����·�Ӧ�ɵõ�����þ��������þ��ˮ������Ӧ����Mg��OH��2��NH3����������������Ʊ�����þ��ʵ�鷽��ʾ���ͼ��ʵ��ǰϵͳ�ڿ������ų���ͼ�м�ͷ��ʾ�������������Ϊ�˷����Ƿ���ȷ����˵������ ��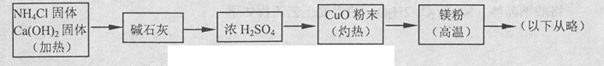
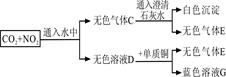
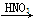 Mg(NO3)2
Mg(NO3)2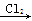 MgCl2
MgCl2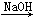 Mg(OH)2
Mg(OH)2 Mg(NO3)2
Mg(NO3)2 MgO
MgO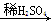 MgSO4
MgSO4 Mg(NO3)2
Mg(NO3)2