��Ŀ����
��ѧʵ��Ҫ���ϡ���ɫ��ѧ�����ijʵ��С��ԡ�ͭ��Ũ���ᷴӦ��������̽����ʵ�顣�Իش���������:
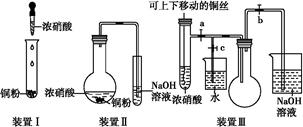
(1)д��ͭ��Ũ���ᷴӦ�����ӷ���ʽ: ��
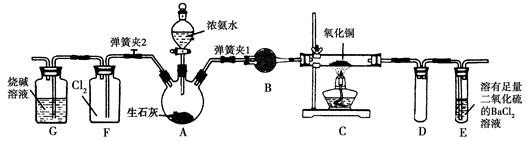
(2)��װ�â����,װ�â���ŵ��� ,װ�â������װ�â���ŵ���,���е��ŵ��� ��
(3)��װ�â���,��ʹNO2���������ƿ,Ӧ�ȹرյ��ɼ���������,�ٴ��ɼ���������;�����������ƿ��,��ͭ˿����,Ȼ���a��b��c���ر�,������ƿ���ڷ�ˮ��,������������������������
E.ѹǿ
(4)Ϊ����֤NO2��ˮ�ķ�Ӧ,��ʹ�ձ��е�ˮ������ƿ�IJ����� ��

(1)д��ͭ��Ũ���ᷴӦ�����ӷ���ʽ: ��
(2)��װ�â����,װ�â���ŵ��� ,װ�â������װ�â���ŵ���,���е��ŵ��� ��
(3)��װ�â���,��ʹNO2���������ƿ,Ӧ�ȹرյ��ɼ���������,�ٴ��ɼ���������;�����������ƿ��,��ͭ˿����,Ȼ���a��b��c���ر�,������ƿ���ڷ�ˮ��,������������������������
| A����ɫ |
| B������ |
| C�������ƽ����Է������� |
| D���ܶ� |
(4)Ϊ����֤NO2��ˮ�ķ�Ӧ,��ʹ�ձ��е�ˮ������ƿ�IJ����� ��
(1)Cu+4H++2N
 Cu2++2NO2��+2H2O
Cu2++2NO2��+2H2O
(2)�����������ݳ�,�����˶Կ�������Ⱦ���ɿ��Ʒ�Ӧ��ʱ����,��ʱֹͣ
(3)c��ab��BD
(4)�ر�a��c,��b,��������ƿˮԡ����,�����岻�ٷų�ʱ�ر�b,ֹͣ����,��c

 Cu2++2NO2��+2H2O
Cu2++2NO2��+2H2O(2)�����������ݳ�,�����˶Կ�������Ⱦ���ɿ��Ʒ�Ӧ��ʱ����,��ʱֹͣ
(3)c��ab��BD
(4)�ر�a��c,��b,��������ƿˮԡ����,�����岻�ٷų�ʱ�ر�b,ֹͣ����,��c
(2)�Ƚ�����װ�õĽṹ,��ϱ�ʵ��ԭ��,װ�â��װ�â���Խ�ĵط���:�����������ݳ�,�����˶Կ�������Ⱦ;װ�â���ŵ㻹��:�ɿ��Ʒ�Ӧ��ʱ����,��ʱֹͣ��
(3)��Ҫ��NO2������ƿ,��Ҫ�رտ���c����a��b;��������ƿ�д���2NO2(g) N2O4(g)����H<0,�ڷ�ˮ��,ƽ������,��ɫ�������ƽ����Է���������С,ѹǿ����,���ǻ��������������ܶȲ��䡣
N2O4(g)����H<0,�ڷ�ˮ��,ƽ������,��ɫ�������ƽ����Է���������С,ѹǿ����,���ǻ��������������ܶȲ��䡣
(4)��Ҫ���ձ��е�ˮ������ƿ,��Ӧ��С��ƿ�ڵ���ѹ,���Կɲ�ȡ��ʩ:�ر�a��c,��b,����ƿˮԡ
����,��һ����NO2�������NaOH��Һ���з�Ӧ,�ٽ���,����ƿ����ѹ���С,��ʱ��c,�ر�b,ˮ��������ƿ��
(3)��Ҫ��NO2������ƿ,��Ҫ�رտ���c����a��b;��������ƿ�д���2NO2(g)
 N2O4(g)����H<0,�ڷ�ˮ��,ƽ������,��ɫ�������ƽ����Է���������С,ѹǿ����,���ǻ��������������ܶȲ��䡣
N2O4(g)����H<0,�ڷ�ˮ��,ƽ������,��ɫ�������ƽ����Է���������С,ѹǿ����,���ǻ��������������ܶȲ��䡣(4)��Ҫ���ձ��е�ˮ������ƿ,��Ӧ��С��ƿ�ڵ���ѹ,���Կɲ�ȡ��ʩ:�ر�a��c,��b,����ƿˮԡ
����,��һ����NO2�������NaOH��Һ���з�Ӧ,�ٽ���,����ƿ����ѹ���С,��ʱ��c,�ر�b,ˮ��������ƿ��

��ϰ��ϵ�д�
�����Ŀ

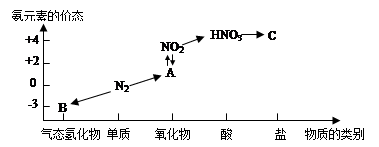
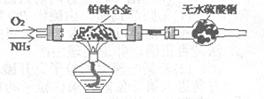
 3Cu+N2+3H2O������������þ�ڸ����·�Ӧ�ɵõ�����þ��������þ��ˮ������Ӧ����Mg��OH��2��NH3����������������Ʊ�����þ��ʵ�鷽��ʾ���ͼ��ʵ��ǰϵͳ�ڿ������ų���ͼ�м�ͷ��ʾ�������������Ϊ�˷����Ƿ���ȷ����˵������ ��
3Cu+N2+3H2O������������þ�ڸ����·�Ӧ�ɵõ�����þ��������þ��ˮ������Ӧ����Mg��OH��2��NH3����������������Ʊ�����þ��ʵ�鷽��ʾ���ͼ��ʵ��ǰϵͳ�ڿ������ų���ͼ�м�ͷ��ʾ�������������Ϊ�˷����Ƿ���ȷ����˵������ ��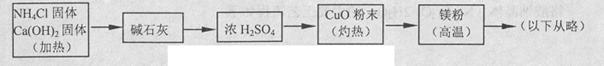

 2NH3��g�� ��H��0��������������ʱ�����¶ȣ�ƽ��ʱ����ת��������
2NH3��g�� ��H��0��������������ʱ�����¶ȣ�ƽ��ʱ����ת��������