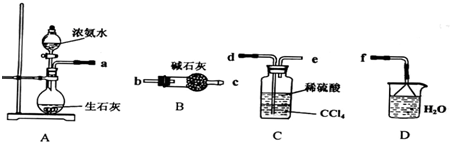
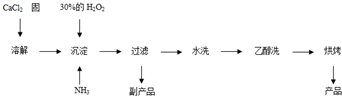
��Ŀ����
��15�֣�ij��ȤС�����ӹ�ҵʳ���о������Σ�������NaCl��Һ����ش��������⣺
��1������ˮ���������A��B�����ʣ�������A��Դ��ʯ��Ҥ������д��A��B�Ļ�ѧʽ��
A B
��2��ʵ�����ᴿ���ε�ʵ���������Ϊ��
ȡ���� �������� �� ����ȴ�ᾧ����ɡ�
��3��ʵ��������500 mL 0.1mol/L��NaCl��Һ�������������Ϊ��������ƽ�ϳ�ȡһ��������NaCl�����������ձ��У�������������ˮʹ����ȫ�ܽ⣻�ڰ��Ƶõ���ҺС�ĵ�ע��500 mL������ƿ�У��ۼ���������ƿ�еμ�����ˮ��Һ���̶���1��2 cm��ʱ�����ý�ͷ�ι�С�ĵμӣ�ֱ����Һ�İ�Һ��ǡ����̶�������Ϊֹ��������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ�ε�ϴ��Һ��ת������ƿ�У�������ҡ�ȣ��ݽ�����ƿ���ã����ҡ�ȡ�����д���пհס�
��Ӧ�ó�ȡ��NaCl������Ϊ________��������������ȷ˳����____________��
�ڱ�ʵ���õ��������У���������_____________________________________________
________________________________________________________________________��
�۹۲�����ƿ�е�Һ��ʱ�������ӿ̶��ߣ���ʹ�������Һ��Ũ��________(�ƫ�ߡ���ƫ�͡�����Ӱ�족����ͬ)��û�н��в����ܣ���________��
��1��Ca(OH)2��CaO��Na2CO3����ÿ��1�֣�
��2���ܽ⣻���ˣ���������ÿ��1�֣� ��3����2.9 g�� �٢ڢܢۢ�
��������ƽ��ҩ�ס��ձ���500 mL����ƿ����ͷ�ιܡ���ƫ�ߡ�ƫ�͡���ÿ��1�֣�
����

 ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�