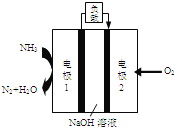
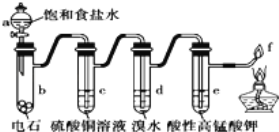
��Ŀ����
����Ŀ����������(FeC2O4)����������������Ӱ���Լ����͵�ز�����������﮵�������ij����������Ʒ(�����ᾧˮ)�к����������ᣬ���õζ����ⶨ����Ʒ��FeC2O4�ĺ�����ʵ�鷽�����£�
�ٽ�0.20 g����������Ʒ����250 mL��ƿ�ڣ���������2 mol��L��1��H2SO4��Һ��ʹ��Ʒ�ܽ⣬������70 �����ң������ø��������Һ�ζ����յ㡣
����ζ��յ���Һ�м���������Zn�ۺ�����2 mol��L��1��H2SO4��Һ�����5��8 min����KSCN��Һ�ڵ�ΰ��ϼ������Һ��ֱ����Һ����죬�����������һ����ƿ�У���0.020 00 mol��L��1�ĸ�����ر���Һ�ζ�����Һ���յ㣬���ĸ�����ر�Һ6.00 mL��
�Իش��������⣺
(1)������ر�Һ��________�ζ���ʢװ(������ʽ��������ʽ��)��
(2)�ڲ�����У��μӸ��������Һʱ�۲쵽����ɫ���������������������ᷴӦ�����ӷ���ʽΪ______________________________________________________��
(3)�ζ��������۾�Ӧע��__________________ ���ζ��յ������� ___________________
(4)�ڲ�����У����в���������ⶨ���ƫ�ߵ���_____________��
a �ζ�����ʢװ�������ǰδ��ϴ
b �ζ������У���ƿ��̫���ң����²���Һ�彦��
c �ζ�ǰ������ȷ���ζ��յ�ʱ���Ӷ���
d �ζ�ǰ���������ݣ��ζ���������ʧ
(5)0.20 g��Ʒ��FeC2O4����������Ϊ____��(����3λ��Ч���֣������Dz�����е����)
���𰸡���ʽ 2MnO4��+ 5H2C2O4+6H+= 2Mn2++ 10CO2��+ 8H2O ��ƿ����Һ��ɫ�仯 ���������һ�α�Һ����Һ��Ϊdz��ɫ���Ұ�����ڲ���ɫ a d 43.2%
��������
��1�����������Һ����ǿ�����ԣ���Ҫʢ������ʽ�ζ����У�
��2��������У��μӸ��������Һʱ�۲쵽����ɫ���������������������Һ��������Ϊ������̼������ԭ���غ�͵���غ���ƽ��д���ӷ���ʽ��
��3���ζ��������۾�Ӧע����ƿ����Һ��ɫ�ı仯�����������һ�α�Һ����Һ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��Ϊ�յ㣻
��4���ζ�ʵ�����������������c������Һ��=![]() �����жϣ����������ı�Һ������仯������ϵ��
�����жϣ����������ı�Һ������仯������ϵ��
��5�����ݵζ������е����ݺ����ӷ���ʽ�Ķ�����ϵ����õ���
��1�����������Һ�������Ժ�ǿ����ʴ��ʽ�ζ��ܵĽ��ܣ�Ӧ����ʽ�ζ���ʢ�ţ�
�ʴ�Ϊ����ʽ��
��2������������H2SO4��Һ��Ӧ���ɲ����������������������������������Һ��Ӧ�����ӷ���ʽ�в���д��������ʽ��������������ᷴӦ�����ӷ���ʽΪ2MnO4��+ 5H2C2O4+6H+= 2Mn2++ 10CO2��+ 8H2O��
�ʴ�Ϊ��2MnO4��+ 5H2C2O4+6H+= 2Mn2++ 10CO2��+ 8H2O��
��3���ζ��������۾�Ӧע����ƿ����Һ��ɫ�ı仯�����������һ�α�Һ����Һ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��Ϊ�յ㣬
�ʴ�Ϊ����ƿ����Һ��ɫ�仯�����������һ�α�Һ����Һ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
��4��a���ζ�����ʢװ�������ǰδ��ϴ����ɸ��������Һ��Ũ�Ƚ��ͣ�������Һ������ζ����ƫ�ߣ���ȷ��
b������Һ�彦���ᵼ�½��ƫ�ͣ�����
c���ζ��յ�ʱ���Ӷ�������ʹĩ������ֵƫС�����ƫ�ͣ�����
d���ζ�ǰ���������ݣ��ζ���������ʧ�����ƫ�ߣ���ȷ��
��ѡad��
��5����KSCN��Һ�ڵ�ΰ��ϼ������Һ��ֱ����Һ����죬˵����Һ�в���Fe3+����0.02000 mol/L�ĸ�����ر���Һ�ζ�����Һ���������ӷ���ʽΪ��MnO4��+ 5Fe2++8H+= Mn2++ 5Fe3++ 4H2O����ȥ������ص����ʵ���Ϊ6��10-3L��0.02000 mol/L�����������������Ϊ6��10-3L��0.02000 mol/L��5��144g/mol=0.0864g��
����Ʒ�� FeC2O4����������Ϊ![]() ��100%=43.2%��
��100%=43.2%��
�ʴ�Ϊ��43.2%��

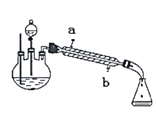
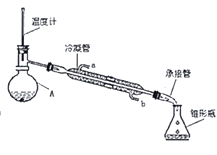
����Ŀ�����ñ���ȩ(C6H5CHO)��NaOH��Һ�п����Ʊ��״�(C6H5CH2OH)�ͱ�����(C6H5COOH)��ʵ��������ͼ��

������ʵ��������±���ʾ���ش��������⣺
���� | ��ˮ������ܶ� | �е� | �ܽ��� |
���״� | 1.04 | 205.7 | ����ˮ������������ |
������ | 1.27 | 249 | ������ˮ����������ˮ������������ |
���� | 0.71 | 34.6 | ��ˮ�������� |
��1���������У���Ҫ�õ��IJ����������ձ��⣬����____�������ٺ��״��������л�����_____(����������������)�㡣
��2�����Ѳ���10��Na2CO3��Һϴ�ӵ�Ŀ����_____��������ǰ����Ҫ����MgSO4��������______�������ڵ�װ����ͼ��ָ����װ��ͼ����������______��

��3����������______����������______��
��4��ȡ106.00g����ȩ��Ӧ��������ȩ��ת����Ϊ80�������ѵ���ȡ��Ϊ100����ϴ�ӵȹ�����ʧ��Ϊ10���������տ��Ƶñ��״�___g(�������С�������λ)��