��Ŀ����
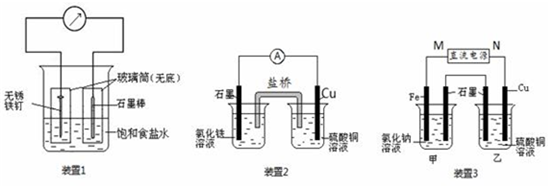
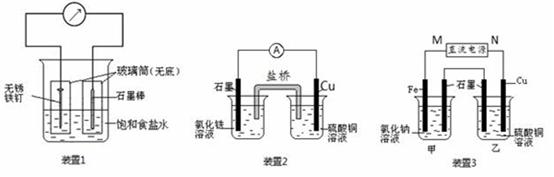
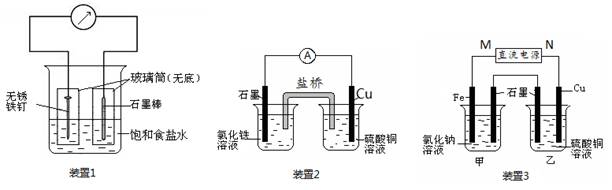
��ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⡣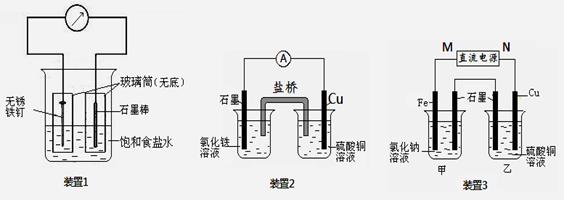
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��� �����̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ ��
��2��װ��2�е�ʯī�� ������������� ������װ�÷������ܷ�Ӧ�����ӷ���ʽΪ ��
��3��װ��3�м��ձ�ʢ��200 mL 0.2 mol/L��NaCl��Һ�����ձ�ʢ��200 mL 1.0 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣����ձ��е��뼸�η�̪��Һ���۲쵽ʯī�缫�������ȱ�졣
|
��12�֣���1��O2+ 4e- + 2H2O = 4OH- ��2������ 2Fe3+ + Cu = 2Fe2+ + Cu2+
��3���� ���� Fe - 2e-= Fe2+ �� 448
���������������1��װ��1Ϊ����������ʴ����̼���������������õ����ӣ�����OH��������̼����Χ��Һ�Լ��ԣ���Һ��죬�õ缫��ӦΪO2+ 4e- + 2H2O = 4OH-��
��2��ͭ�ǽ�����ʯī�Ƿǽ���������ʯī����������Һ�е������ӵõ����ӡ�ͭ�Ǹ�����ʧȥ���ӣ�����װ��2�е��ܷ�Ӧʽ��2Fe3+ + Cu = 2Fe2+ + Cu2+��
��3��������ձ��е��뼸�η�̪��Һ���۲쵽ʯī�缫�������ȱ�죬˵����ʯī�缫������OH�����ӣ���ʯī�����������������������Ե�Դ��M��Ϊ�����������缫�ĵ缫��ӦΪFe - 2e-= Fe2+��
��Cu�缫��ϴ�ӡ�����������缫���� 1.28 g����˵����������ͭ��������1.28g�����ʵ�����0.02mol��ת��0.04mol�����Ը��ݵ��ӵ�ʧ�غ��֪���������������ʵ�����0.04mol��2��0.02mol���ڱ�״���µ������448ml��
���㣺����绯ѧӦ�õ��й��жϡ��缫��Ӧʽ����д�Լ��йؼ���
�����������Ǹ߿��г������ͣ������е��Ѷȵ����⡣���������ǿ�����ض�ѧ����������������ⷽ������������ʱ��Ҫע��缫���жϺ͵缫��Ӧ����д��ע�����·�и��缫ת�Ƶĵ�����Ŀ��ȣ����÷�Ӧ�ķ���ʽ���㡣

 ��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ
��
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ
��