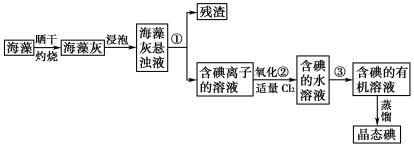
��Ŀ����
����Ŀ�����÷Ͼ�п��Ƥ�Ʊ�����Fe3O4�������Ӽ�������ZnO��һ���Ʊ�����ͼ���£�

��֪��Ksp[Zn(OH)2]= 1.2��10��17��Zn(OH)2��������ǿ�ᣬ��������ǿ��������ڰ�ˮ������[Zn��NH3)4]2����
��1����ҺA�м�ϡH2SO4����Zn(OH)2�����ӷ���ʽΪ_________��
��2�������£�Zn(OH)2������Һ��c(Zn2��)=3��10��6mol/L������ҺA�м���ϡH2SO4���������ܽ������Zn(OH)2��Zn(OH)2��ʼ�ܽ��pHΪ_________��Ϊ��ֹZn(OH)2�ܽ⣬�ɽ�ϡH2SO4��Ϊ_________����lg2=0.3��
��3���������������Σ�NaClO3����ԭΪCl������ԭ������������Ӧ�����ʵ���֮����_________��
��4��������ҺB�Ƶ�Fe3O4�������ӵĹ�����ͨ��N2��ԭ����_________��
��Fe3O4�������ӵ�ֱ���ķ�Χ��_________��
��ȷ����ҺB�к���Fe2�����Լ���_________��
��5���Խ�����ʵ���Ҳ������ÿ�����п���백ˮ��Ӧ�Ʊ�������п��ԭ��_________��
���𰸡� ZnO22�� + 2H+ = Zn(OH)2�� 8.3 CO2 6:1 ��ֹFe2+[��Fe(OH)2]�������������������� 1��100nm K3[Fe(CN)6]��Һ(�����軯����Һ�����Ը�����أ� ������п���백ˮ��Ӧ������������пҪ���ڹ����İ�ˮ�У�����[Zn(NH3)4]2+����ˮ���������ƣ�ֻ�����������п�����ڹ����İ�ˮ��Ҳ�����֣���
���������Ͼɶ�п��Ƥ��������������Һ�з�Ӧ��п�ܽ�����ƫп���ƺ������������ܽ⣬���˵õ���ҺAΪNa2ZnO2��������ΪFe����ҺA��ϡ����ʹ��Һ��ZnO22-ת��ΪZn(OH)2�������پ������ˡ�ϴ�ӡ�������յõ�ZnO��������Fe�м���ϡ���ᣬ��Ӧ�����Ȼ���������������NaClO3������������������Ϊ�����ӣ��õ���Fe2+��Fe3+��B��Һ���ټ���NaHCO3����ͨ�뵪�������������������������ӡ�
(1)��ҺA ΪNa2ZnO2����ϡH2SO4����Zn(OH)2�����ӷ���ʽΪZnO22�� + 2H+ = Zn(OH)2�����ʴ�Ϊ��ZnO22�� + 2H+ = Zn(OH)2����
(2)�����£�Zn(OH)2������Һ��c(Zn2��)=3��10��6mol/L����c(OH-)=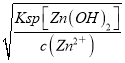 =
=![]() =2��10��6mol/L��pH=14-lg(2��10��6)=8.3Ϊ��ֹZn(OH)2�ܽ⣬�ɽ�ϡH2SO4��Ϊ���ᣬ��ͨ�������̼���ʴ�Ϊ��8.3��CO2��
=2��10��6mol/L��pH=14-lg(2��10��6)=8.3Ϊ��ֹZn(OH)2�ܽ⣬�ɽ�ϡH2SO4��Ϊ���ᣬ��ͨ�������̼���ʴ�Ϊ��8.3��CO2��
(3)�����м���NaClO3����������������Ϊ�����ӣ�������ӦΪ6Fe2++ClO3-+6H+=6Fe3++Cl-+3H2O����ԭ��Ϊ�������ӡ�������Ϊ�����ƣ���ԭ���������������ʵ���֮��6:1���ʴ�Ϊ��6:1��
(4)�ٷ�ֹFe2+������������ҺB�Ƶ�Fe3O4�������ӵĹ�����ͨ��N2���ʴ�Ϊ����ֹFe2+��������
��Fe3O4�������ӵ�ֱ���ķ�Χ��1��100nm���ʴ�Ϊ��1��100nm ��
�ۼ����������ӿ����仹ԭ�ԣ�����Ϊ��ȡ����B��Һ���μ�KMnO4��Һ��(��)��ɫ��ȥ���ʴ�Ϊ��KMnO4��Һ��
(5)������п���백ˮ��Ӧ������������пҪ���ڹ����İ�ˮ�У�����[Zn(NH3)4]2+����ˮ���������ƣ������ʵ���Ҳ������ÿ�����п���백ˮ��Ӧ�Ʊ�������п���ʴ�Ϊ��������п���백ˮ��Ӧ������������пҪ���ڹ����İ�ˮ�У�����[Zn(NH3)4]2+����ˮ������������

 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�����Ŀ����ȥ����������������������(������Ϊ����)����ѡ�õ��Լ��ͷ��뷽���ܴﵽʵ��Ŀ�ĵ���
����� | �Լ� | ���뷽�� | |
A | CO2(SO2) | ����Na2CO3��Һ | ϴ�� |
B | ����(��ϩ�� | ���� | ���� |
C | ��������(���ᣩ | NaOH��Һ | ���� |
D | ����(�Ȼ��ƣ� | ����ˮ | ���� |
A. A B. B C. C D. D