��Ŀ����
����Ŀ�����й������ʵ���Ũ�ȱ�����ȷ����
A��0.3 mol��L1��Na2SO4��Һ�к���Na+��![]() �������ʵ���Ϊ0.9 mol
�������ʵ���Ϊ0.9 mol
B����1 Lˮ����22.4 L����ʱ���ð�ˮ��Ũ�Ȳ���1 mol��L1��ֻ�е�22.4 L��������ˮ�Ƶ�1 L��ˮʱ����Ũ�Ȳ���1 mol��L1
C����K2SO4��NaCl�����Ի��ˮ��Һ�У����Na+��![]() �����ʵ�����ȣ���K+��Cl�����ʵ���Ũ��һ�����
�����ʵ�����ȣ���K+��Cl�����ʵ���Ũ��һ�����
D��10 ��ʱ��0.35 mol��L1��KCl������Һ100 mL������5 gˮ����ȴ��10 ��ʱ�������С��100 mL���������ʵ���Ũ����Ϊ0.35 mol��L1
���𰸡�D
��������A�û��ָ����Һ�����������Һ��Na+��![]() �������ʵ�����һ������0.9 mol��B���Ȼǿ���˰�ˮ�����Ϊ1 L���������ܼ�Ϊ1 L������û��ָ��22.4 L�ǰ����ڱ�״���µ���������������ʵ�����һ����1 mol�����Ƴ�1 L��Һʱ��Ũ��Ҳ��һ����1 mol��L1��C���Na+��
�������ʵ�����һ������0.9 mol��B���Ȼǿ���˰�ˮ�����Ϊ1 L���������ܼ�Ϊ1 L������û��ָ��22.4 L�ǰ����ڱ�״���µ���������������ʵ�����һ����1 mol�����Ƴ�1 L��Һʱ��Ũ��Ҳ��һ����1 mol��L1��C���Na+��![]() �����ʵ������ʱ�����ݻ�ѧʽ��K+��
�����ʵ������ʱ�����ݻ�ѧʽ��K+��![]() ��Na+��Cl�ı�����ϵ���ɵ�c(K+)��c(Cl)=2��1��D�����10 ��ʱ0.35 mol��L1��KCl������Һ��������ˮ�ֺ�����KCl���壬�¶Ȼָ���10 ��ʱ����Ϊ������Һ����Ũ�Ȳ��䡣
��Na+��Cl�ı�����ϵ���ɵ�c(K+)��c(Cl)=2��1��D�����10 ��ʱ0.35 mol��L1��KCl������Һ��������ˮ�ֺ�����KCl���壬�¶Ȼָ���10 ��ʱ����Ϊ������Һ����Ũ�Ȳ��䡣

 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д�����Ŀ���ȱ���Ⱦ�ϡ�ҽҩ��ҵ���й㷺��Ӧ�ã�ijʵ��С����������װ�úϳ��ȱ���֧���õ�����̨����ʡ�ԣ���ͨ��һ�������ᴿ�ȱ���
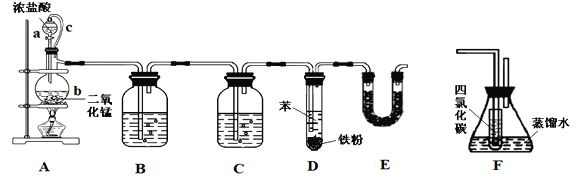
��Ӧ��Ͳ������������б����£�
�ܶ�/g��cm��3 | �е�/�� | ˮ���ܽ��� | |
�� | 0.879 | 80.1 | �� |
�ȱ� | 1.11 | 131.7 | ���� |
�밴Ҫ��ش��������⡣
��1��װ��A����c��������______________��װ��E��������__________________��
��2��ʵ��ʱ��ʹa�е�Ũ���Ỻ�����£��ɹ۲쵽����b�ڵ�������________________��д����Ӧ�����ӷ���ʽ______________________________________��
��3��Ϊ֤�������ͱ���������ȡ�������Ǽӳɷ�Ӧ����С����װ��F˵������װ��F����________֮�䣨����ĸ����F��С�Թ���CCl4��������___________________������ʹ�õ��Լ���______________��
��4����֪D�м���5 mL���������ᴿ���ռ����ȱ�3.0 g�����ȱ��IJ���Ϊ_________%��������λ��Ч���֣���



