��Ŀ����
����Ŀ��һ���¶��£����ݻ�ΪV L�ĺ����ܱ�������ͨ��1 mol CH3Cl(g)��1 mol H2O(g)����ϵ��ͬʱ������������ƽ�⣺
��Ӧ�٣�CH3Cl(g)��H2O(g)![]() CH3OH(g)��HCl(g) K1
CH3OH(g)��HCl(g) K1
��Ӧ�ڣ�2CH3OH(g)![]() (CH3)2O(g)��H2O(g) K2
(CH3)2O(g)��H2O(g) K2
��Ӧt min����ϵ�ﵽƽ�⣬��ʱ(CH3)2O(g)�����ʵ���Ϊ9��10-3mol��CH3Cl(g)��ƽ��ת����Ϊ4.8%���ش��������⣺
(1)0��t min�ڣ�CH3Cl(g)�ķ�Ӧ����Ϊ___________��
(2)��Ӧ�ﵽƽ��ʱ��CH3OH(g)�����ʵ���Ϊ___________��
(3)���㷴Ӧ�ڵ�ƽ�ⳣ��K2��___________��
(4)����Ӧ����ƽ��ʱ��������ϵ��ͨ��һ������CH3OH(g)������˵����ȷ����________(����ĸ)��
A.��Ӧ�ٵ�ƽ�������ƶ�����Ӧ�ڵ�ƽ�ⲻ�����ƶ�
B.ƽ��ʱ��Ӧ�١���Ӧ�ڵķ�Ӧ���ʶ�����
C.K1����K2��С
���𰸡�![]() molL-1min-1 0.03mol 9.61 B
molL-1min-1 0.03mol 9.61 B
��������
һ���¶��£����ݻ�ΪV L�ĺ����ܱ�������ͨ��1 mol CH3Cl(g)��1 mol H2O(g)����ϵ��ͬʱ������������ƽ�⣺
��Ӧ�٣�CH3Cl(g)��H2O(g)![]() CH3OH(g)��HCl(g) K1
CH3OH(g)��HCl(g) K1
��Ӧ�ڣ�2CH3OH(g)![]() (CH3)2O(g)��H2O(g) K2
(CH3)2O(g)��H2O(g) K2
��Ӧ�ﵽƽ��ʱҪ���ǵ�CH3OH(g)��Ũ����������ѧ��Ӧ�ۺϺ�õ��Ľ����
��1����Ӧ��ʼʱ��CH3Cl(g)Ϊ1 mol��t min��CH3Cl(g)��ƽ��ת����Ϊ4.8%������CH3Cl(g)�����ʵ����仯��Ϊ1mol![]() 4.8%=0.048mol����ѧ��Ӧ����=
4.8%=0.048mol����ѧ��Ӧ����=![]() =
=![]() =
=![]() molL-1min-1���ʴ�Ϊ
molL-1min-1���ʴ�Ϊ![]() molL-1min-1��
molL-1min-1��
��2����ϵ��������Ӧͬʱ�������ɷ�Ӧ��CH3Cl(g)�����ʵ����仯��Ϊ0.048mol����֪������CH3OH(g)�����ʵ���Ϊ0.048mol���ɷ�Ӧ����(CH3)2O(g)�����ʵ���Ϊ9��10-3mol����֪���ĵ�CH3OH(g)�����ʵ���Ϊ9��10-3mol��2=0.018mol�����Է�Ӧ�ﵽƽ��ʱ��CH3OH(g)�����ʵ���Ϊ0.048mol-0.018mol=0.03mol���ʴ�Ϊ0.03mol��
��3����Ӧ�ٴﵽƽ���û�вμӷ�Ӧ��H2O(g)�����ʵ���Ϊ1mol-0.048mol=0.952mol����Ӧ�����ɵ�H2O��g�������ʵ���Ϊ9��10-3mol������ƽ��ʱ������H2O��g�������ʵ���Ũ��Ϊ![]() =
=![]() molL-1��(CH3)2O(g)�����ʵ���Ũ��Ϊ
molL-1��(CH3)2O(g)�����ʵ���Ũ��Ϊ![]() =
=![]() molL-1���ɣ�2����֪ƽ��ʱCH3OH(g)�����ʵ���Ũ��Ϊ
molL-1���ɣ�2����֪ƽ��ʱCH3OH(g)�����ʵ���Ũ��Ϊ![]() =
=![]() molL-1��ƽ�ⳣ��K2��
molL-1��ƽ�ⳣ��K2��![]() =9.61���ʴ�Ϊ9.61��
=9.61���ʴ�Ϊ9.61��
��4������Ӧ����ƽ��ʱ��������ϵ��ͨ��һ������CH3OH(g)��CH3OH(g)���ʵ���Ũ������Ӧ�ٵ�ƽ�������ƶ�����Ӧ�ڵ�ƽ�������ƶ���ƽ��ʱ��Ӧ�١���Ӧ�ڵķ�Ӧ���ʶ������¶Ȳ���ʱ����Ӧ��ƽ�ⳣ�������ı䣬�ʴ�ѡB��

 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�����Ŀ��̼���γɻ�������������Ԫ�أ��䵥�ʼ��γɵĻ����������������������Ҫ��Դ���ʡ�
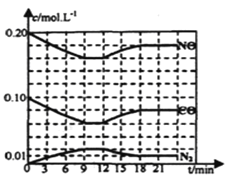
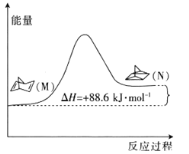
(1)�л���M����̫������տ�ת��������N���������仯��ͼ��ʾ����M��N��ȣ����ȶ�����________(����M������N��)��
(2)��֪��
C(s)��H2O(l)��CO(g)��H2(g) ��H1��a kJ��mol-1
2CO(g)��O2(g)��2CO2(g) ��H2��b kJ��mol-1
2H2(g)��O2(g)��2H2O(l) ��H3��c kJ��mol-1
��C(s)��O2(g)��CO2(g) ��H��______(��a��b��c��ʾ)kJ��mol-1��
(3)���ݼ������ݹ���CH4(g)��4F2(g)��CF4(g)��4HF(g)�ķ�Ӧ����H��_________��
��ѧ�� | C-H | C-F | H-F | F-F |
����(KJmol-1) | 414 | 489 | 565 | 155 |

(4)��һ���ݵ��ܱ������У�����1 mol CO(g)��2 mol H2O(g)��������ӦCO(g)��H2O(g)![]() H2(g)��CO2(g) ��H��CO��ƽ��ת�������¶ȵı仯��ͼ��ʾ��
H2(g)��CO2(g) ��H��CO��ƽ��ת�������¶ȵı仯��ͼ��ʾ��
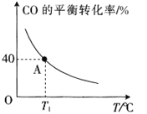
�ٸ÷�Ӧ����H________(����<������>��)0��
�����������ʱ��Ҫ����÷�Ӧ������Ӧ���ʿɲ�ȡ�Ĵ�ʩ��_________(��дһ��)��
��A��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ___________(��ȷ��0.01)��
����Ŀ�������������л��ʵ������֮���ת����ѡ�Լ�������������ȷ���ǣ� ��
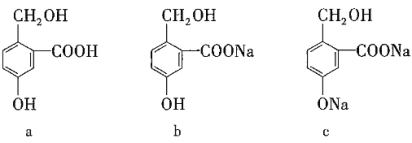
ѡ�� | aת��Ϊb | aת��Ϊc | cת��Ϊb |
A | NaOH | Na | CO2 |
B | Na2CO3 | NaOH | HCl |
C | NaHCO3 | NaOH | CO2 |
D | NaHCO3 | NaCl | HCl |
A.AB.BC.CD.D
����Ŀ����������ʵ���ܵó���Ӧ���۵���
ѡ�� | ʵ�� | ���� |
A | ������,���0.1mol/LNaA��Һ��pHС��0.1mol/L Na2CO3��Һ��pH | ����:HA>H2CO3 |
B | ���е��۵�FeI2��Һ�м���������ˮ,��Һ����ɫ | ��ԭ��:I->Fe2+ |
C | ��FeSO4��Һ�м���CuS����,�����Һ��c(Fe2+)���� | Ksp(CuS)<Ksp(FeS) |
D | ��ˮ�еμ�����AgNO3��Һ���������� | Ag+��NH3��H2O�ܴ������� |
A. A B. B C. C D. D