��Ŀ����
����Ŀ����ѧ֪ʶ����������ϢϢ��ء�
I.�輰�仯����
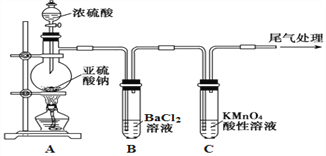
��1���輰�仯�������ִ���Ϣ������Ӧ�ù㷺������������������оƬ�IJ�����__________��д��ѧʽ��������������ά�IJ�����__________��д��ѧʽ�������ά�ڼ����������ױ���ʴ����д����ص����ӷ�Ӧ����ʽ______________________
II.�ȼ��仯����
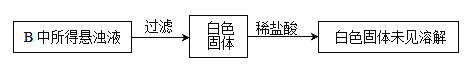
��2��Ư�����������Ʒ������Ҫ�ɷ���__________________��д��ѧʽ����Ư�۱��治���ױ��ʣ���д��Ư��ʧЧ�ķ���ʽ________________���粻С�İ�Ư�������飨��Ҫ�ɷ�Ϊ���ᣩ��ϣ�����������ʹ���ж�����д���йط�Ӧ�����ӷ���ʽ________________________��
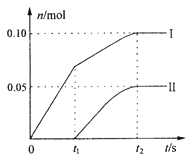
��3��ijʵ��С�飬��һ������ʯ�����л������ٵ�ͨ���������������ֲ����л�������ClO3�����������ۺ���Ϊ���Ƿ�Ӧ�����¶����ߵ�ԭ��������ClO����ClO3���������ӵ����ʵ�����n���뷴Ӧʱ��(t)�Ĺ�ϵ���ߣ����Ա�ʾΪ��ͼ��������������ˮ�ķ�Ӧ����
��ͼ������I��ʾ_____________���ӵ����ʵ����淴Ӧʱ��仯�Ĺ�ϵ��
����ȡʯ�����к���Ca(OH)2�����ʵ���Ϊ__________mol��
����ȡһ����ڵ����ʵ�����ʯ���飬�Խϴ������ͨ��������������Ӧ���ò�����Cl�������ʵ���Ϊ0.37mol���������n(ClO��)/n(ClO3��)=______________��
III.�����仯����
��4�����������������ŷų����ķ�������NO��NO2�ȴ�����Ⱦ�
�ٹ�ҵ�ϳ���ԭ����NOx+NH3��N2+H2O��ʹ��ת��Ϊ����N2������NO��NO2�Ļ��3.0L������3.5L��ͬ״����NH3��ȫ��Ӧ��ȫ��ת��ΪN2������ԭ���������NO��NO2�����ʵ���֮����_____________��
����֪���ܳ�ȥ����β����2NO2+2NaOH��NaNO2+NaNO3+H2O;NO+NO2+2NaOH��2NaNO2+H2O
��������β�������ķ�Ӧԭ�������������в��ܱ�����NaOH��Һ���յ���_______________
A��1molO2��4molNO2��
B��1molO2��4molNO��
C��1molNO��5molNO2�� ��
D��4molNO��1molNO2
��5�������ŷŵ�β�������е������������Ⱦ��������ɲ����������������Ҫԭ����_____________
A��ȼ�պ���������ȼ�ϡ�������������
B��ȼ�պ�Ǧ����
C������ȼ�ղ���֡�����������������
D����ȼ���п����е�N2�ڸ����±�����
���𰸡� Si SiO2 SiO������2OH��=SiO32-+H2O CaCl2��Ca(ClO)2 Ca(ClO)2����CO��+H2O=2HClO+CaCO3�� 2HClO![]() 2HCl��O2�� ClO- ��Cl-��2H+=Cl2 ��+H2O ClO- 0.25 7��6 1:3 D D
2HCl��O2�� ClO- ��Cl-��2H+=Cl2 ��+H2O ClO- 0.25 7��6 1:3 D D
����������1�������Ͷ����������;�����þ���������õİ뵼�壬�����������оƬ�IJ����Ǿ���裬��ѧʽΪSi�����ά�ijɷ���SiO2��SiO2��������������������ֽⷴӦ����SiO2��2OH��=SiO32����H2O����2������Ư�۵ijɷֺ�ʧЧ��ԭ��Ư����Ca(ClO)2��CaCl2�Ļ���̼�������ǿ�ڴ����ᣬ�����Ca(ClO)2��CO2��H2O=CaCO3��2HClO��HClO�������ȷֽ⣬2HClO![]() 2HCl��O2�������߷���������ԭ��Ӧ�����������������ӷ�Ӧ����ʽΪClO����Cl����2H��=H2O��Cl2������3������������ԭ��Ӧ�е�ʧ������Ŀ�غ㣬�ٸ������⣬����ClO3����ԭ���Ƿ�Ӧ�Ƿ��ȣ��¶�������ɵģ�˵����Ӧ��ʼʱû��ClO3�������ɣ���I������ΪClO����II������ΪClO3�����ڷ�Ӧ���ɵIJ�����CaCl2��Ca(ClO)2��Ca(ClO3)2��H2O��t2ʱ��ʱ������ClO3����ClO�������ʵ���Ϊ0.05mol��0.10mol�����ݵ�ʧ������Ŀ�غ㣬���n(Cl��)=n(ClO��)��5n(ClO3��)����n(Cl��)=( 0.10��5��0.05)mol=0.35mol�����Ca(OH)2�����ʵ���Ϊ[n(Cl��)��n(ClO��)��n(ClO3��)]/2=(0.05��0.1��0.35)/2mol=0.25mol���۸��ݵ�ʧ������Ŀ�غ㣬��n(Cl��)=n(ClO��)��5n(ClO3��)=0.37mol��Ca(OH)2�����ʵ���Ϊ[n(Cl��)��n(ClO��)��n(ClO3��)]/2=0.25mol�����n(ClO��)=0.07mol��n(ClO3��)=0.06mol�������n(ClO��)/n(ClO3��)=7/6����4������������ԭ��Ӧ�е�ʧ������Ŀ�غ㣬�Լ���ѧ���㣬����ͬ״���£���������ȵ��������ʵ���֮�ȣ���NO�����ΪxL����NO2�����Ϊ(3��x)L�����ݵ�ʧ������Ŀ�غ㣬��2x��(3��x)��4=3��3.5�����x=0.75L��NO2�����Ϊ2.25L�����߱�ֵΪ0.75��2.25=1��3����A������4NO2��O2��2H2O=4HNO3��NO2��O2ǡ����ȫ��Ӧ������HNO3��Ȼ��HNO3��NaOH��Ӧ������ȫ�������գ���A����B����2NO��O2=2NO2���������㣬�����������м��㣬����2molNO2��ʣ��2molNO��������Ϣ��2molNO2��2molNO��������������Һ��ȫ�������գ���B����C�����������������Ӧ����ʽ������ȫ�����գ���C����D������������Ӧ����ʽ��NO���������岻�ܱ���ȫ���գ���D��ȷ����5�����黷����Ⱦ����Դ������β���е������������ɿ����еĵ����ڸ�������������Ӧ���ɣ���ѡ��D��ȷ��
2HCl��O2�������߷���������ԭ��Ӧ�����������������ӷ�Ӧ����ʽΪClO����Cl����2H��=H2O��Cl2������3������������ԭ��Ӧ�е�ʧ������Ŀ�غ㣬�ٸ������⣬����ClO3����ԭ���Ƿ�Ӧ�Ƿ��ȣ��¶�������ɵģ�˵����Ӧ��ʼʱû��ClO3�������ɣ���I������ΪClO����II������ΪClO3�����ڷ�Ӧ���ɵIJ�����CaCl2��Ca(ClO)2��Ca(ClO3)2��H2O��t2ʱ��ʱ������ClO3����ClO�������ʵ���Ϊ0.05mol��0.10mol�����ݵ�ʧ������Ŀ�غ㣬���n(Cl��)=n(ClO��)��5n(ClO3��)����n(Cl��)=( 0.10��5��0.05)mol=0.35mol�����Ca(OH)2�����ʵ���Ϊ[n(Cl��)��n(ClO��)��n(ClO3��)]/2=(0.05��0.1��0.35)/2mol=0.25mol���۸��ݵ�ʧ������Ŀ�غ㣬��n(Cl��)=n(ClO��)��5n(ClO3��)=0.37mol��Ca(OH)2�����ʵ���Ϊ[n(Cl��)��n(ClO��)��n(ClO3��)]/2=0.25mol�����n(ClO��)=0.07mol��n(ClO3��)=0.06mol�������n(ClO��)/n(ClO3��)=7/6����4������������ԭ��Ӧ�е�ʧ������Ŀ�غ㣬�Լ���ѧ���㣬����ͬ״���£���������ȵ��������ʵ���֮�ȣ���NO�����ΪxL����NO2�����Ϊ(3��x)L�����ݵ�ʧ������Ŀ�غ㣬��2x��(3��x)��4=3��3.5�����x=0.75L��NO2�����Ϊ2.25L�����߱�ֵΪ0.75��2.25=1��3����A������4NO2��O2��2H2O=4HNO3��NO2��O2ǡ����ȫ��Ӧ������HNO3��Ȼ��HNO3��NaOH��Ӧ������ȫ�������գ���A����B����2NO��O2=2NO2���������㣬�����������м��㣬����2molNO2��ʣ��2molNO��������Ϣ��2molNO2��2molNO��������������Һ��ȫ�������գ���B����C�����������������Ӧ����ʽ������ȫ�����գ���C����D������������Ӧ����ʽ��NO���������岻�ܱ���ȫ���գ���D��ȷ����5�����黷����Ⱦ����Դ������β���е������������ɿ����еĵ����ڸ�������������Ӧ���ɣ���ѡ��D��ȷ��