��Ŀ����
�������о������ʣ���H2 ���� ��CuO ��CO2 ��ϡH2SO4 ��ϡ���� �߰�ˮ ��Ba(OH)2���� ������Al2(SO4)3������Żش��������⡣
��1�����������У����ڼ���� ��
��2���������ʻ���Һ����������Ӧ�����ӷ���ʽΪ��H+��OH��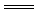 H2O�����Ӧ������������ ��
H2O�����Ӧ������������ ��
��3���������ʻ�����Һ��������Ӧ�������������Ӧ���������ʵ���Ϸֱ��� ��
��4��17.1g������ˮ���250mL��Һ�� SO42-�����ʵ���Ũ��Ϊ ��
��5���ڢ����Һ��ͨ������Ģܣ�д���ܷ�Ӧ�����ӷ���ʽ ��
��6�������������£����������Ӧ�Ļ�ѧ����ʽΪ��8Al + 30HNO3 = 8Al(NO3)3 +3X + 9H2O���÷�Ӧ��X�Ļ�ѧʽ�� �����л�ԭ���������������ʵ���֮���� ������8.1g Al������Ӧʱ��ת�Ƶ��ӵ����ʵ���Ϊ ��
��1�����������У����ڼ���� ��
��2���������ʻ���Һ����������Ӧ�����ӷ���ʽΪ��H+��OH��
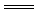 H2O�����Ӧ������������ ��
H2O�����Ӧ������������ ����3���������ʻ�����Һ��������Ӧ�������������Ӧ���������ʵ���Ϸֱ��� ��
��4��17.1g������ˮ���250mL��Һ�� SO42-�����ʵ���Ũ��Ϊ ��
��5���ڢ����Һ��ͨ������Ģܣ�д���ܷ�Ӧ�����ӷ���ʽ ��
��6�������������£����������Ӧ�Ļ�ѧ����ʽΪ��8Al + 30HNO3 = 8Al(NO3)3 +3X + 9H2O���÷�Ӧ��X�Ļ�ѧʽ�� �����л�ԭ���������������ʵ���֮���� ������8.1g Al������Ӧʱ��ת�Ƶ��ӵ����ʵ���Ϊ ��
��1���� ��2���͢� ��3���ں͢ݣ��ں͢� ����1�֣� ��4��0.6 mol��L-1��
��5��OH����CO2��HCO3������6��NH4NO3��8��3��0.9mol��
��5��OH����CO2��HCO3������6��NH4NO3��8��3��0.9mol��
�����������1����ˮ�Լ��ԣ�����ˮ�����ڼ���ǻ�����2�����ӷ���ʽΪ��H+��OH��=H2O��������ǿ����ǿ�Ӧ���ɿ������ε��кͷ�Ӧ����3����Ӧ����������������������뷴Ӧ������һ�����ʿ������ᣨ������⣩��Ҳ������ǿ���4������c="n/v" ���ɵ�SO42-�����ʵ���Ũ��Ϊ
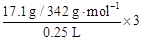 ="0.6" mol/L ����5����Ba(OH)2����Һ��ͨ�������CO2�������ɳ�������������ܽ⣬����������ʽ�Σ��ܷ�Ӧ�����ӷ���ʽOH����CO2��HCO3������6������Hԭ���غ㣬X�к���HԪ�أ���֪XΪ��Σ�XΪNH4NO3�����ɵ����غ���֤���ɣ���ԭ�������������������ᣬ����Nԭ�ӵı�۸�����֪����3������õ��ӣ���ԭ���������������ʵ���֮���� 8 : 3����8.1g Al������Ӧʱ����ʧȥ���ӵ����ʵ���Ϊ
="0.6" mol/L ����5����Ba(OH)2����Һ��ͨ�������CO2�������ɳ�������������ܽ⣬����������ʽ�Σ��ܷ�Ӧ�����ӷ���ʽOH����CO2��HCO3������6������Hԭ���غ㣬X�к���HԪ�أ���֪XΪ��Σ�XΪNH4NO3�����ɵ����غ���֤���ɣ���ԭ�������������������ᣬ����Nԭ�ӵı�۸�����֪����3������õ��ӣ���ԭ���������������ʵ���֮���� 8 : 3����8.1g Al������Ӧʱ����ʧȥ���ӵ����ʵ���Ϊ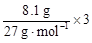 ="0.9" mol��
="0.9" mol��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��
�� ��
�� ��
�� ��Ϊͬ��������
��Ϊͬ�������� ��NH4Cl�ĵ���ʽΪ
��NH4Cl�ĵ���ʽΪ
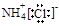

 H2CO3+H3O+
H2CO3+H3O+