��Ŀ����
̼���������ĵ��ʼ��仯�����ڹ�ũҵ����������������Ҫ���á�
��1�����̼�Ȼ�ԭһ�Ȼ�����ʵ���������Ʊ�������������ص��Ȼ�ѧ����ʽ���£�
2Al2O3��s��+ 2AlCl3��g��+ 6C��s����6AlCl��g��+ 6CO��g������H�� a kJ?mol-1
3AlCl��g���� 2Al��l��+ AlCl3��g������H�� b kJ?mol-1
��ӦAl2O3��s��+ 3C��s���� 2Al��l��+ 3CO��g���ġ�H�� kJ?mol-1
���ú�a��b�Ĵ���ʽ��ʾ����
��2���û���̿��ԭ�����Դ����������ij�о�С����ij�ܱ������м���һ�����Ļ���̿��NO��������ӦC��s��+ 2NO��g��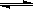 N2��g��+ CO2��g������H= Q kJ?mol-1����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2��g��+ CO2��g������H= Q kJ?mol-1����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
| ʱ�䣨min�� Ũ�ȣ�mol/L�� | 0 | 10 | 20 | 30 | 40 | 50 |
| NO | 1��00 | 0��68 | 0��50 | 0��50 | 0��60 | 0��60 |
| N2 | 0 | 0��16 | 0��25 | 0��25 | 0��30 | 0��30 |
| CO2 | 0 | 0��16 | 0��25 | 0��25 | 0��30 | 0��30 |
��0��10min�ڣ�NO��ƽ����Ӧ����v��NO��= ��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K=
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����ϱ��е������жϸı������������ ������ĸ��ţ�
a��ͨ��һ������NO b������һ�����Ļ���̿
c��������ʵĴ��� d���ʵ���С���������
����30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ3��1��1����Q 0���������������
���ں��ݾ��������£����жϸ÷�Ӧһ���ﵽ��ѧƽ��״̬�������� ����ѡ���ţ�
a����λʱ��������2nmol NO��g����ͬʱ����nmol CO2��g��
b����Ӧ��ϵ���¶Ȳ��ٷ����ı�
c�����������ܶȲ��ٷ����ı�
��1�� (1��)
(1��)
��2���� 0��032 mol/(L��min) ��1�֣� 0��25��2�֣�
�� a d ��2�֣���ѡ��ѡ�����֣�©ѡ��1�֣�
�� ����2�֣�
�� b c ��2�֣���ѡ��ѡ�����֣�©ѡ��1�֣�
���������������1�����ݸ�˹���ɣ���ӦAl2O3��s��+ 3C��s���� 2Al��l��+ 3CO��g�����з���ʽ��1����1/2+��2���ã���˴������Ӧ�ʱ�ֵ����a/2+b����2����0��10min�ڣ�NO��ƽ����Ӧ����v��NO��=��1-0��68��/10=0��032 mol/(L��min)��T1��ʱ����20minʱ�����Ũ�Ȳ��ٷ����仯��˵����Ӧ�ﵽƽ�⣬�ɵ�ƽ�ⳣ��Ϊ��0��25��0��25��/0��52=0��25����30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����Ũ�ȶ����ӣ���������С���������NO������̼�ǹ��壬����������ƽ�ⲻ�ƶ������ѡad������30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ3��1��1�����ԭ����2:1��1��ƽ�������ƶ���NO�������ӣ����������¶ȣ�ƽ�������ȷ�Ӧ�����ƶ�������Ӧ�Ƿ��ȷ�Ӧ��Q<0�����ں��ݾ��������£����жϸ÷�Ӧһ���ﵽ��ѧƽ��״̬��a����λʱ��������2nmol NO��g������Ӧ���淴Ӧ������У�����nmol CO2��g����Ҳ����������У����ܱ�ʾƽ�⣬����b����Ӧ��ϵ���¶Ȳ��ٷ����ı䣬��Ϊ�Ǿ�����ϵ�����Ե��¶Ȳ��ٸı�ʱ����ʾ�ﵽƽ�⣬��ȷ�� c�����������ܶȲ��ٷ����ı䣬������䣬��̼�ǹ��壬�������������ٸı�ʱ��Ҳ�����ܶȲ��䣬�ﵽƽ�⣬��ȷ��
���㣺�����˹���ɡ���ѧ��Ӧԭ��

��ο�����ͼ������֪E1��134 kJ��mol��1��E2��368 kJ��mol��1������Ҫ��ش����⣺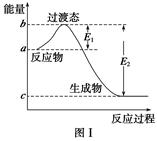 ��
��
��1��ͼ����1 mol NO2(g)��1 mol CO(g)��Ӧ����CO2��NO�����е������仯ʾ��ͼ�����ڷ�Ӧ��ϵ�м����������Ӧ��������E1�ı仯��________(���������С�����䡱����ͬ)����H�ı仯��________����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��________________________________��
��2���״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧ���Ȼ�ѧ����ʽ���£�
��CH3OH(g)��H2O(g)=CO2(g)��3H2(g)��H����49��0 kJ��mol��1
��CH3OH(g)�� O2(g)=CO2(g)��2H2(g)��H����192��9 kJ��mol��1
O2(g)=CO2(g)��2H2(g)��H����192��9 kJ��mol��1
��֪��H2O(g)=H2O(l)����H����44 kJ��mol��1����״�����ȼ��ΪҺ̬ˮ���Ȼ�ѧ����ʽΪ______________________��
��3�������ʾ�Dz��ֻ�ѧ���ļ��ܲ�����
| ��ѧ�� | P��P | P��O | O=O | P=O |
| ����/kJ��mol��1 | a | b | c | x |
��֪����ȼ����Ϊd kJ��mol��1����������ȫȼ�յIJ���Ľṹ��ͼ����ʾ�������x��________ kJ��mol��1(�ú�a��b��c��d�Ĵ�����ʽ��ʾ)��
̼���仯�����й㷺����;��
(1)��ˮ����ͨ�����ȵ�̼���ɲ���ˮú������ӦΪ
C(s)��H2O(g) CO(g)��H2(g)����H����131.3 kJ��mol��1��
CO(g)��H2(g)����H����131.3 kJ��mol��1��
���Ϸ�Ӧ�ﵽƽ������������������£����´�ʩ���������H2O��ƽ��ת���ʵ���________��(�����)
| A�������¶� | B������̼������ | C��������� | D����CO���ռ���ȥCO |
 2CO(g)����H����172.5 kJ��mol��1����CO(g)��H2O(g)
2CO(g)����H����172.5 kJ��mol��1����CO(g)��H2O(g) CO2(g)��H2(g)���ʱ䦤H��________��
CO2(g)��H2(g)���ʱ䦤H��________��(3)CO��H2��һ�������¿ɷ�Ӧ���ɼ״���CO(g)��2H2(g)
 CH3OH(g)���״���һ��ȼ�ϣ������ü״����һ��ȼ�ϵ�أ���ϡ�������������Һ�����ʯī���缫���õ�ظ�����ӦʽΪ__________________________________��
CH3OH(g)���״���һ��ȼ�ϣ������ü״����һ��ȼ�ϵ�أ���ϡ�������������Һ�����ʯī���缫���õ�ظ�����ӦʽΪ__________________________________�����øõ���ṩ�ĵ��ܵ��60 mL NaCl��Һ������0.01 mol CH3OH��ȫ�ŵ磬NaCl�������ҵ�������Cl2ȫ���ݳ������ǰ�������Һ����ı仯�����������������Һ��pH��________��
(4)��һ������CO(g)��H2O(g)�ֱ�ͨ�뵽���Ϊ2.0 L�ĺ����ܱ������У��������·�Ӧ��CO(g)��H2O(g)
 CO2(g)��H2(g)���õ��������ݣ�
CO2(g)��H2(g)���õ��������ݣ�| �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| H2O | CO | H2 | CO | | |
| 900 | 1.0 | 2.0 | 0.4 | 1.6 | 3.0 |
ͨ����������÷�Ӧ��ƽ�ⳣ��(���������λ��Ч����)________���ı䷴Ӧ��ijһ��������Ӧ���е�t minʱ����û��������CO2�����ʵ���Ϊ0.6 mol������200 mL 5 mol/L��NaOH��Һ������ȫ���գ���Ӧ�����ӷ���ʽΪ(��һ�����ӷ���ʽ��ʾ)__________________________
(5)��ҵ�����ǰ�ˮú���еĻ�����徭���������õĽϴ�H2���ںϳɰ����ϳɰ���Ӧԭ��ΪN2(g)��3H2(g)
 2NH3(g)����H����92.4 kJ��mol��1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�������·�Ӧ��N2Ũ����ʱ��仯��ͼ����ʾ��
2NH3(g)����H����92.4 kJ��mol��1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�������·�Ӧ��N2Ũ����ʱ��仯��ͼ����ʾ��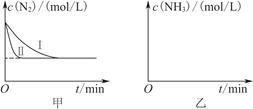
��ش��������⣺
����ʵ���Ƚϣ�ʵ���ı������Ϊ________________________________��
��ʵ����ʵ�����¶�Ҫ�ߣ�����������ͬ������ͼ���л���ʵ����ʵ�����NH3Ũ����ʱ��仯��ʾ��ͼ��
�о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����塣
(1)��CO2�뽹̿��������CO��CO�����������ȡ�
����֪��Fe2O3(s)��3C(ʯī)=2Fe(s)��3CO(g)����H1����489.0 kJ��mol��1
C(ʯī)��CO2(g)=2CO(g)����H2����172.5 kJ��mol��1
��CO��ԭFe2O3���Ȼ�ѧ����ʽΪ___________________________
������ȼ�շ�Ӧ����Ƴ�CO/O2ȼ�ϵ��(��KOH��ҺΪ���Һ)��д���õ�صĸ�����Ӧʽ___________________________________________
(2)ijʵ�齫CO2��H2����һ��������ܱ������У������ֲ�ͬ�����·�Ӧ��
CO2(g)��3H2(g) CH3OH(g)��H2O(g)�� ��H����49.0 kJ��mol��1
CH3OH(g)��H2O(g)�� ��H����49.0 kJ��mol��1
���CH3OH�����ʵ�����ʱ��仯����ͼ��ʾ���ش����⣺
�����д�ʩ����ʹn(CH3OH)/n(CO2)�������________��
| A�������¶� | B������He(g)ʹ��ϵѹǿ���� |
| C����H2O(g)����ϵ�з��� | D���ٳ���1 mol CO2��3 mol H2 |
��һ���¶��£����ݻ���ͬ�ҹ̶��������ܱ������У������·�ʽͶ�뷴Ӧ�һ��ʱ���ﵽƽ�⡣
| ���� | �� | �� |
| ��Ӧ��Ͷ���� | 1 mol CO2��3 mol H2 | a mol CO2��b mol H2��c mol CH3OH(g)��c mol H2O(g) |
������ƽ��������ѹǿΪ��ʼʱ��
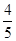 ��Ҫʹƽ������������ͬ��ֵ����������ȣ�����ʼʱά�ַ�Ӧ������У���c��ȡֵ��ΧΪ________��
��Ҫʹƽ������������ͬ��ֵ����������ȣ�����ʼʱά�ַ�Ӧ������У���c��ȡֵ��ΧΪ________��(3)��0.10 mol��L��1����ֱ�ζ�20.00 mL 0.10 mol��L��1��NaOH��Һ��20.00 mL 0.10 mol��L��1��ˮ���õĵζ��������£�
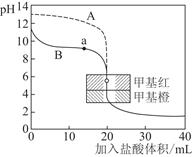
��ָ������ζ���ˮ������Ϊ________(�A����B��)����д������a������Ӧ����Һ�и�����Ũ���ɴ�С������˳��________��
���û�ѧ��Ӧԭ���о�NH3�����ʾ�����Ҫ���塣��ش��������⣺
��1���������������Թ���ȼ�ϵ�أ����ط�Ӧԭ��Ϊ4NH3��3O2=2N2��6H2O����������ҺӦ���� ������ԡ������ԡ����ԡ����������ĵ缫��ӦʽΪ ��
��2��25��ʱ����amol��L��1�İ�ˮ��0.1mol��L��1������������ϡ�
�ٵ���Һ������Ũ�ȹ�ϵ����c(NH4+)>c(Cl-)��ʱ����Ӧ���������Ϊ ��
A������㣮��ˮʣ�� B����ˮ������ǡ����ȫ��Ӧ C���������
�ڵ���Һ��c(NH4+)=c(Cl-)��ʱ���ú���a���Ĵ���ʽ��ʾNH3��H2O�ĵ���ƽ�ⳣ��Kb=______________.
��3����0.5L�����ܱ������У�һ������N2��H2���з�Ӧ��N2(g)��3H2(g) 2NH3(g) ?H=bkJ/mol���仯ѧƽ�ⳣ��K���¶ȵĹ�ϵ���£�
2NH3(g) ?H=bkJ/mol���仯ѧƽ�ⳣ��K���¶ȵĹ�ϵ���£�
| �¶�/�� | 200 | 300 | 400 |
| K | 1.0 | 0.86 | 0.5 |
��д���÷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽ��__________��b________������ڡ���С�ڡ����ڡ���0
��400��ʱ�����ijʱ�̰��������������������ʵ����ֱ�Ϊ3mol��2mol��1molʱ����ʱ�̸÷�Ӧ��v����N2��_________������ڡ���С�ڡ����ڡ���v����N2��.
��4����֪����4NH3(g)��3O2(g)=2N2(g)��6H2O(g) ?H="-1266.8KJ/mol" ����N2(g)��O2(g)=2NO(g) ?H=+180.5KJ/mol��д�������´��������Ȼ�ѧ����ʽ�� ��
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã����ٵ����������ڴ����е��ŷ��ǻ�����������Ҫ����֮һ��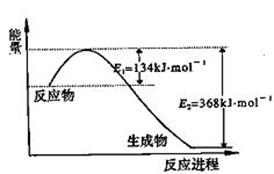
��1����ͼ��1 mol NO2�����1 mol CO���巴Ӧ����CO2�����NO��������������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ�� ��
��֪��N2 (g)+2NO2 (g)  4NO(g) ��H=+292.3kJ��mol��1��
4NO(g) ��H=+292.3kJ��mol��1��
��Ӧ�� 2NO(g)+2CO(g) N2(g)+2CO2(g) �ġ�H= ��
N2(g)+2CO2(g) �ġ�H= ��
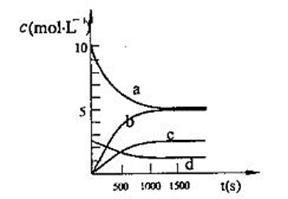
��2��һ���¶��£������Ϊ2L�ĺ����ܱ������г���20 mol NO2��5 mol O2������Ӧ�� 4NO2(g)+O2(g) 2N2O5(g)����֪��ϵ��n(NO2)��ʱ��仯���±���
2N2O5(g)����֪��ϵ��n(NO2)��ʱ��仯���±���
| t(s) | 0 | 500 | 1000 | 1500 |
| n(NO2)(mol) | 20 | 13.96 | 10.08 | 10.08 |
��д���÷�Ӧ��ƽ�ⳣ������ʽ��K= ����֪��K3000C��K3500C����÷�Ӧ�� ��Ӧ(����ȡ������ȡ�)��
�ڷ�Ӧ�ﵽƽ���NO2��ת����Ϊ ����Ҫ����NO2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��
A�������¶�
B�����뺤����ʹ��ϵѹǿ����
C���ٳ���NO2
D���ٳ���4 mol NO2��1 mol O2
��ͼ�б�ʾN2O5��Ũ�ȵı仯������ ����O2��ʾ��0~500s�ڸ÷�Ӧ��ƽ������v= ��

 O2(g)=CO2(g)�� ��H2��b kJ��mol��1
O2(g)=CO2(g)�� ��H2��b kJ��mol��1 2NH3(g)����H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol(�����1��1)����5 L�ϳ����У���ӦǰѹǿΪp0����Ӧ������ѹǿ��p��ʾ����Ӧ������
2NH3(g)����H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol(�����1��1)����5 L�ϳ����У���ӦǰѹǿΪp0����Ӧ������ѹǿ��p��ʾ����Ӧ������ ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
 O2��g����H2 O��g�� ��H����241.8kJ��mol��1
O2��g����H2 O��g�� ��H����241.8kJ��mol��1 2NO��g�� ��H
2NO��g�� ��H 0����1.0 mol������0.80 mol N2��0.20 mol O2��1300oCʱ��1.0 L�ܱ������ھ���5s��Ӧ�ﵽƽ�⣬���NOΪ8.0��10��4 mol��
0����1.0 mol������0.80 mol N2��0.20 mol O2��1300oCʱ��1.0 L�ܱ������ھ���5s��Ӧ�ﵽƽ�⣬���NOΪ8.0��10��4 mol�� 2CO2��g����N2��g�� �У�NO��Ũ��
2CO2��g����N2��g�� �У�NO��Ũ��