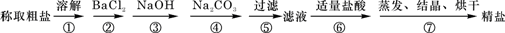��Ŀ����
��12�֣�ÿ��2�֣����к�NaCl��Na2SO4��NaNO3�Ļ���ѡ���ʵ����Լ�����ת��Ϊ��Ӧ�ij�������壬�Ӷ�ʵ��Cl-��SO42-����NO3-������롣��Ӧ��ʵ����̿�����ͼ��ʾ��
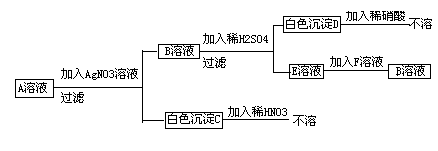
 ��ش��������⣺
��ش��������⣺
[1]д��ʵ���������������ʵĻ�ѧʽ
�Լ�X �� ����A�� ����B��
[2]����ʵ�������м��������Na2CO3��Ŀ���� _____ ��
[3]д���۵Ļ�ѧ����ʽ����________________________________________
[4]����ʵ�鷽���õ�����Һ3�п϶����� ���ѧʽ�����ʡ�
 ��ش��������⣺
��ش��������⣺[1]д��ʵ���������������ʵĻ�ѧʽ
�Լ�X �� ����A�� ����B��
[2]����ʵ�������м��������Na2CO3��Ŀ���� _____ ��
[3]д���۵Ļ�ѧ����ʽ����________________________________________
[4]����ʵ�鷽���õ�����Һ3�п϶����� ���ѧʽ�����ʡ�
��ÿ�ո�2�֣���12�֣���BaCl2 [��Ba(NO3)2] BaSO4 AgCl
�Ƴ�ȥ������Ba2+��Ag+
[3]NaCl+AgNO3=AgCl+NaNO3 ���������ɣ� [4]Na2CO3
�Ƴ�ȥ������Ba2+��Ag+
[3]NaCl+AgNO3=AgCl+NaNO3 ���������ɣ� [4]Na2CO3
�������ʵķ������ᴿ��
��1�����ݲ���ڿ�֪��B���Ȼ��������Բ�����е��Լ����Ȼ�������A�����ᱵ��
��2����Һ2�к���Ag����Ba2��������̼���Ƶ������dz�ȥ��Һ�е�Ag����Ba2����
��3����Ӧ�ڵĻ�ѧ����ʽ��NaCl+AgNO3=AgCl+NaNO3��
��4��̼�����ǹ����ģ�������Һ3��һ������̼���ơ�
��1�����ݲ���ڿ�֪��B���Ȼ��������Բ�����е��Լ����Ȼ�������A�����ᱵ��
��2����Һ2�к���Ag����Ba2��������̼���Ƶ������dz�ȥ��Һ�е�Ag����Ba2����
��3����Ӧ�ڵĻ�ѧ����ʽ��NaCl+AgNO3=AgCl+NaNO3��
��4��̼�����ǹ����ģ�������Һ3��һ������̼���ơ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
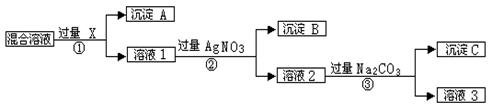
�����Ŀ

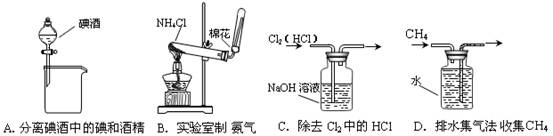
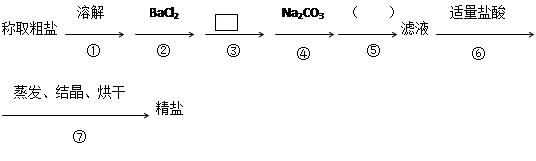 ��1���ж�BaCl2�ѹ����ķ����� ��
��1���ж�BaCl2�ѹ����ķ����� �� ����д��ʹ�ó����Լ��Ļ�ѧʽ__________���ڣ����еIJ���������____ ��
����д��ʹ�ó����Լ��Ļ�ѧʽ__________���ڣ����еIJ���������____ ��