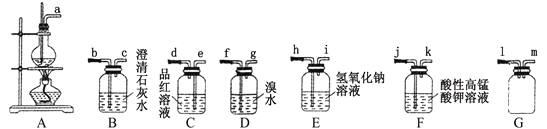
��Ŀ����
����Ŀ��Ϊ��֤����ʵ�����Ʊ�Cl2�Ĺ����л���ˮ������HCl�ӷ���������ͬѧ���������ͼ��ʾ��ʵ��װ�ã���Ҫ��ش����⡣����ʾ����ˮ����ͭ��ˮ����������������CCl4���л��ܼ���
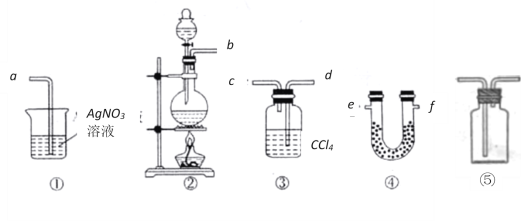
(1)���ݼ�ͬѧ����ͼ��������Ӧ��װ�ã��ӿ�˳��Ϊ��b��___��____��_____��____��a��
(2)�����Ӻ�װ��֮��ʵ�鿪ʼ֮ǰ����Ҫ���е�һ������ǣ�___________________��
(3)U�ι�����ʢ�Լ��Ļ�ѧʽΪ___________��װ�â���CCl4��������____________��
(4)��ͬѧ��Ϊ��ͬѧʵ����ȱ�ݣ�����֤������ͨ��AgNO3��Һ�е�����ֻ��һ�֡�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��AgNO3��Һ������ֻ��һ�֣���ͬѧ�����ij����װ��֮
���ټ�װ�âݣ�����Ϊװ�â�Ӧ����__________________֮�䣨��װ����ţ���ƿ�п��Է���_________________________________��
(5)����װ����õ�ǰ���£���Ũ��Ϊ10mol/L��Ũ����600mL������ĵ�MnO2��Ӧ���������ɵ�����_____1.5mol������ڡ�С�ڻ���ڣ�������Ҫԭ����___________________________������ͨ����Ӧ�����Һ�м���___________��֤���������ۡ�
A��п�� B������������Һ C�������ữ����������Һ D��̼������Һ
���𰸡�e f d c ���װ�������� CuSO4 �������� �٢� ʪ��ĵ��۵⻯����ֽ С�� ���ŷ�Ӧ�Ľ��У�����Ũ�ȱ�ϡ����Ӧֹͣ A D
��������
Ϊ��֤����ʵ�����Ʊ�Cl2�Ĺ����л���ˮ������HCl�ӷ�����������ѡ����ȡ������װ�âڣ�Ȼ��������ˮ����ͭ����ˮ������ʹ��װ�âܣ����л��ܼ����Ȼ�̼��ȥ������ʹ��װ�â������װ�â��е������������Ȼ��⡣�ݴ˻ش����⡣�������̺�Ũ���ᷴӦ������������ϡ�����Ӧ��
(1) Ϊ��֤����ʵ�����Ʊ�Cl2�Ĺ����л���ˮ������HCl�ӷ�������ѡ����ȡ������װ��Ϊ�ڣ�Ȼ��������ˮ����ͭ����ˮ������ʹ��װ�âܣ����л��ܼ����Ȼ�̼��ȥ������ʹ��װ�â������װ�â��е������������Ȼ���.���Ե��ܿڵ�����˳��Ϊbefdca��
(2) ���Ӻ�װ��֮��ʵ�鿪ʼ֮ǰ����Ҫ���װ�������ԡ�
(3)U�����Լ� ΪCuSO4��װ�â���CCl4����������������
(4)��Ϊ�����Ȼ�̼������������û��֤���Ƿ�������ȫ��������Ҫ�ڢ٢�֮�����һ������������װ�ã���ʹ��ʪ��ĵ��۵⻯����ֽ������ֽ����������֤������������ȫ��ͨ���������в�����ɫ��������֤���Ȼ�����ڡ�
(5) ���ŷ�Ӧ�Ľ��У�����Ũ�ȱ�ϡ����Ӧֹͣ������600mL 10mol/L��Ũ���ᷴӦ���ɵ�����С��1.5mol��������Ӧ�����Һ�м���п��̼����֤����Һ�к���ʣ����ᣬ��ѡA D��