��Ŀ����
����Ŀ����ǹ�ҵ���Ʊ�Na2S2O3�ķ���֮һ����Ӧԭ��Ϊ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2���÷�Ӧ��>0��ij�о�С����ʵ��������Ʊ�Na2S2O3��5H2O�������¡�

��1������װ����ͼ��ʾ��

��װ��B�������Ǽ���װ����SO2������Ч�ʣ�B���Լ���________������SO2����Ч�ʵ͵�ʵ��������B����Һ________________��
��Ϊ��ʹSO2������������ȫ���ڲ��ı�A����ҺŨ�ȡ�����������£����˼�ʱ���跴Ӧ���⣬���ɲ�ȡ�ĺ�����ʩ��_______��_______����д��������
��2�����豾ʵ�����õ�Na2CO3������NaCl��NaOH�����ʵ�鷽�����м��顣������ʱCaCO3������Һ��pH=12��, �����Լ���������ϡ���ᡢAgNO3��Һ��CaCl2��Һ��Ca(NO3)2��Һ����̪��Һ������ˮ��pH�ơ��ձ����Թܡ��ιܡ�
��� | ʵ����� | Ԥ������ | ���� |
�� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ⣬_________�� | �а�ɫ�������� | ��Ʒ��NaCl |
�� | ��ȡ������Ʒ���ձ��У�������������ˮ��������ܽ⣬_________�� | �а�ɫ�������ɣ��ϲ���ҺpH>10.2 | ��Ʒ��NaOH |
��3��Na2S2O3��Һ�Ƕ���ʵ���еij����Լ����ⶨ��Ũ�ȵĹ������£�
��һ����ȷ��ȡag KIO3����Է���������214�����������Һ��
�ڶ������������KI�����H2SO4��Һ���μ�ָʾ����
����������Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��Һ��Һ�����ΪvmL ��c(Na2S2O3��Һ)��_______mol��L-1����ֻ�г���ʽ���������㣩
��֪��IO3-��I-��6H+=3I2+3H2O ��2S2O32-��I2=S4O62-��2I- ijͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�Na2S2O3��Ũ�ȿ���_____�������Ӱ�족����ƫ�͡���ƫ�ߡ�) ��ԭ����________�������ӷ���ʽ��ʾ����
���𰸡� Ʒ�졢��ˮ������KMnO4��Һ ��Һ��ɫ�ܿ���ɫ�������������𰸣� ����SO2������ �ʵ������¶ȣ������������𰸣� �μ�����ϡ���ᣬ�ٵμ�����AgNO3��Һ���� �������CaCl2��Һ�����裬���ã���pH�Ʋ����ϲ���ҺpH 6000a/214V��3000a/107V ƫ�� O2+4H++4I-=2I2+2H2O
��������(1)������������л�ԭ�ԡ�Ư���ԣ����Կ�����Ʒ�졢��ˮ��KMnO4��Һ����������������Ƿ���ȫ���գ���SO2����Ч�ʵͣ������������ʣ�࣬B�е���Һ����ɫ���ʴ�Ϊ��Ʒ�졢��ˮ��KMnO4��Һ����Һ��ɫ�ܿ���ɫ����Ϊ��ʹSO2������������ȫ���ڲ��ı�A����ҺŨ�ȡ�����������£����Լ���������������٣�ʹ������������Һ��ֽӴ���Ӧ���ʵ������¶ȣ�Ҳ��ʹ���������ַ�Ӧ���ʴ�Ϊ������SO2�����٣��ʵ������¶ȣ�(2)ʵ�����õ�Na2CO3������NaCl��NaOH��������NaCl���ڣ����ȼ�ϡ�����ų����ţ��ټ���������Һ�����а�ɫ�������ɣ�˵����NaCl����֪����ʱCaCO3������Һ��pH=10.2����Ҫ�����������ƴ��ڣ���������CaCl2��Һ����Na2CO3ת��ΪCaCO3���ٲ�����Һ��pH����pH����10.2��˵������NaOH���ʴ�Ϊ��
��� | ʵ����� | Ԥ������ | ���� |
�� | �����μ�����ϡ���ᣬ�ٵμ�����AgNO3��Һ���� | ||
�� | �����������CaCl2��Һ�����裬���ã���pH�Ʋⶨ�ϲ���ҺpH�� |
��(3)KIO3+5KI+3H2SO4=3K2SO4+3I2+3H2O��I2+2Na2S2O3=Na2S4O6+2NaI��n(KIO3)= ![]() mol����μӷ�Ӧ��Na2S2O3Ϊxmol�� KIO3��������3I2��������6Na2S2O3
mol����μӷ�Ӧ��Na2S2O3Ϊxmol�� KIO3��������3I2��������6Na2S2O3
1 6
![]() mol xmol
mol xmol
���� x=![]() ����c(Na2S2O3)=
����c(Na2S2O3)= ![]() =
=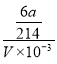 =
=![]() molL-1�������������¿����е�O2Ҳ����KI����ΪI2��ʹ�����ɵĵ�����ʵ���ƫС��ʹ���ĵ�Na2S2O3ƫ�٣��Ӷ�ʹ��õ�Na2S2O3��Ũ��ƫ�ͣ��ʴ�Ϊ��
molL-1�������������¿����е�O2Ҳ����KI����ΪI2��ʹ�����ɵĵ�����ʵ���ƫС��ʹ���ĵ�Na2S2O3ƫ�٣��Ӷ�ʹ��õ�Na2S2O3��Ũ��ƫ�ͣ��ʴ�Ϊ�� ![]() ��ƫ�ͣ�O2+4H++4I-=2I2+2H2O��
��ƫ�ͣ�O2+4H++4I-=2I2+2H2O��

����Ŀ������Cu2O���ھ��������Ĵ����ܶ����ܹ������±�Ϊ��ȡ����Cu2O�����ַ�����
����I | ��̿���ڸ��������»�ԭCuO |
������ | ��ⷨ����ӦΪ2Cu +H2O |
����III | ����(N2H4)��ԭ����Cu(OH)2 |
(1)��ҵ�ϳ��÷�����ͷ�������ȡCu2O�������÷�������ԭ���Ƿ�Ӧ���������ƣ������Ʋ���������________________��ʹCu2O���ʽ���
(2)��֪��2Cu(s)+1/2O2(g)=Cu2O(s������H = -akJ��mol-1
C(s)+1/2O2(g)=CO(g)����H = -bkJ��mol-1
Cu(s)+ 1/2O2(g)=CuO(s)����H = -ckJ��mol-1
�������ķ�Ӧ��2CuO(s)+C(s)= Cu2O(s)+CO(g)����H =_________kJ��mol-1
(3)������������ӽ���Ĥ���Ƶ��Һ��OH-��Ũ�ȶ��Ʊ�����Cu2O��װ������ͼ����������Cu2O����缫��ӦʽΪ___________________��

(4)������Ϊ������������Һ̬��(N2H4)��ԭ����Cu(OH)2���Ʊ�����Cu2O��ͬʱ�ų�N2���÷�Ӧ�Ļ�ѧ����ʽΪ_________________________��
(5)���ֳ�������������ˮ�����백���Ƶ�����õ��뷽��ʽ��ʾ�µ�ˮ��Һ�Լ��Ե�ԭ��___________________��
(6)��1L�����ܱ������г���0.1molN2H4����30�桢Ni-Pt���������·�����ӦN2H4(g) ![]() N2(g)+2H2(g)����û������ϵ�У�
N2(g)+2H2(g)����û������ϵ�У� ![]() (��y��ʾ)��ʱ��Ĺ�ϵ��ͼ��ʾ��0-4minʱ����H2��ƽ����������v(H2)= ____ mol/��L��min�������¶��£���Ӧ��ƽ�ⳣ��=___________________��
(��y��ʾ)��ʱ��Ĺ�ϵ��ͼ��ʾ��0-4minʱ����H2��ƽ����������v(H2)= ____ mol/��L��min�������¶��£���Ӧ��ƽ�ⳣ��=___________________��

����Ŀ��Ϊȷ��Na2CO3��NaHCO3�������Ʒ���������ȡ�ķݸ���Ʒ����ˮ��ֱ���μ�����ͬŨ������30.0 mL����ַ�Ӧ������CO2�����(������ɱ�״���µ������������CO2��ˮ�е��ܽ�)���±���
ʵ����� | �� | �� | �� | �� |
�������(mL) | 30.0 | 30.0 | 30.0 | 30.0 |
��Ʒ����(g) | 2.96 | 3.70 | 5.18 | 6.66 |
CO2���(mL) | 672 | 840 | 896 | 672 |
(1)��Ʒ�е����ʵ���֮��n(Na2CO3)��n(NaHCO3)��________��
(2)��������ʵ���Ũ��c(HCl)��________��
����Ŀ���������ʷ������ȷ����ǣ��� ����
ѡ�� | �� | �� | ���������� | �� |
A | H2CO3 | ���� | CaO | ���� |
B | H2SO4 | �ռ� | Na2O | С�մ� |
C | HNO3 | ��ˮ | Al2O3 | ʯ��ʯ |
D | NaHCO3 | ��ʯ�� | Al��OH��3 | ʳ�� |
A. A B. B C. C D. D


