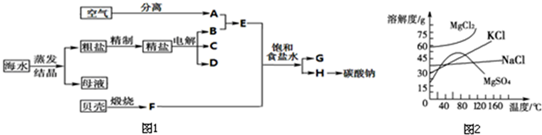
��Ŀ����
18���о���Ա�����������̿���Ҫ�ɷ���MnO2���������᳧��β��SO2���Ʊ������̵������������£�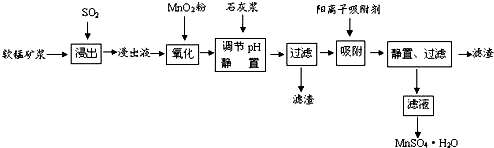
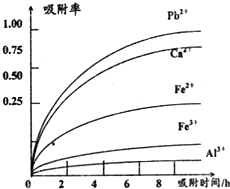
��֪������Һ��pH��2�����еĽ���������Ҫ��Mn2+��������������Fe2+��Al3+��Ca2+��Pb2+���� �����������ӣ�PbO2�������Դ���MnO2��PbSO4��һ�������ʣ��йؽ������ӵİ뾶���γ� �����������ʱ��pH������������������������������ӵ�Ч������ͼ��
| ���� | ���Ӱ뾶��pm�� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 74 | 7.6 | 9.7 |
| Fe3+ | 64 | 2.7 | 3.7 |
| Al3+ | 50 | 3.8 | 4.7 |
| Mn2+ | 80 | 8.3 | 9.8 |
| Pb2+ | 121 | 8.0 | 8.8 |
| Ca2+ | 99 | - | - |
��1��д���������������ɷ�Ӧ�Ļ�ѧ����ʽSO2+MnO2=MnSO4��
��2��������������Ҫ��Ӧ�����ӷ���ʽ2Fe2++MnO2+4H+=2Fe3++Mn2++2H2O��
��3�����������Һ���м���ʯ�ҽ������ڵ���pHֵ���˴�����pHֵ�õ���������pH�ƣ�Ӧ����pH�ķ�ΧΪ4.7��pH��8.3��
��4�������������������ڳ�ȥ���ʽ�������•������ͼ������Ϣ�ش𣬾�������������������Ч��������������ʱ�䡢�������ӵİ뾶���������ӵĵ�ɵȣ����������ȥ����Ҫ����Ϊ��Pb2+��Ca2+��
��5��CaSO4��һ�������ʣ���֪Ksp��CaSO4��=9.10��10-6���ֽ�c mol•L-1CaCl2��Һ��2.00��10-2
mol•L-1Na2SO4��Һ�������� ����������ı仯���������ɳ���ʱ��c����Сֵ��1.82��10-3mol•L-1��
���� ���±��պ������������SO2���������̿���Ҫ�ɷ���MnO2��ͨ��SO2����Һ��pH��2�����еĽ���������Ҫ��Mn2+����MnO2��SO2����������ԭ��Ӧ������Һ������������Fe2+��Al3+�������������ӣ�Fe2+���л�ԭ�ԣ����Ա�MnO2������������������Fe3+�����������Һ���м���ʯ�ҽ��������к���Fe2+��Al3+��Ca2+��Pb2+���������ӣ��ɳ�����pH��Χ֪��Fe2+�ij�����Mn2+���ӵij��������pH�ӽ�����Fe3+������Զ���ʿ��Խ�Fe2+������Fe3+�����ӣ��������ʵ�ͼ���Կ�����Ca2+��Pb2+�������ʽϸߣ���ֻҪ����pHֵ��4.7��8.3�䣬Fe3+��Al3+������ͨ����pHֵ��ת��Ϊ������������������������ͬʱ�����ܵ�����ƣ����ˣ�������Ҫ��������������������������ƣ�������ȡ��MnSO4•H2O���нᾧˮ���ʲ���a��������Ũ���ᾧ�ķ������õ�MnSO4•H2O��
��1��������ͼ�����������������̿�MnO2����SO2�ķ�Ӧ��
��2������������ֻ��Fe2+���л�ԭ�ԣ����Ա�MnO2������������������Fe3+��
��3���ӱ����Կ�����ֻҪ����pHֵ��4.7��8.3�䣬����4.7���Խ�Fe3+��Al3+��ȥ��С��8.3�Ƿ�ֹMn2+Ҳ������
��4����ϰ뾶��������ͼ֪��ͼ�����Ӵ������£��뾶�м�С���ƣ���Ӧ�������ʼ�С������ʱ��ĵ������������ӵ������ʾ�������Fe3+��Al3+����������������������ʵͣ��������ʵ�ͼ���Կ�����Ca2+��Pb2+�������ʽϸߣ�
��5��2.00��10-2 mol•L-1Na2SO4��Һ��c��SO42-��=2.00��10-2 mol•L-1������Ksp��CaSO4��=c��Ca2+��•c��SO42-���������c��Ca2+��������ȷ��c��ֵ��
��� �⣺���±��պ������������SO2���������̿���Ҫ�ɷ���MnO2��ͨ��SO2����Һ��pH��2�����еĽ���������Ҫ��Mn2+����MnO2��SO2����������ԭ��Ӧ������Һ������������Fe2+��Al3+�������������ӣ�Fe2+���л�ԭ�ԣ����Ա�MnO2������������������Fe3+�����������Һ���м���ʯ�ҽ��������к���Fe2+��Al3+��Ca2+��Pb2+���������ӣ��ɳ�����pH��Χ֪��Fe2+�ij�����Mn2+���ӵij��������pH�ӽ�����Fe3+������Զ���ʿ��Խ�Fe2+������Fe3+�����ӣ��������ʵ�ͼ���Կ�����Ca2+��Pb2+�������ʽϸߣ���ֻҪ����pHֵ��4.7��8.3�䣬Fe3+��Al3+������ͨ����pHֵ��ת��Ϊ������������������������ͬʱ�����ܵ�����ƣ����ˣ�������Ҫ��������������������������ƣ�������ȡ��MnSO4•H2O���нᾧˮ���ʲ���a��������Ũ���ᾧ�ķ������õ�MnSO4•H2O��
��1����Ʒλ���̿���Ҫ�ɷ���MnO2��ͨ��SO2����Һ��pH��2�����еĽ���������Ҫ��Mn2+����MnO2��SO2����������ԭ��Ӧ����Ӧ�Ļ�ѧ����ʽΪSO2+MnO2=MnSO4���ʴ�Ϊ��SO2+MnO2=MnSO4��
��2������������ֻ��Fe2+���л�ԭ�ԣ����Ա�MnO2������������������Fe3+����Ӧ�����ӷ���ʽΪ2Fe2++MnO2+4H+=2Fe3++Mn2++2H2O��
�ʴ�Ϊ��2Fe2++MnO2+4H+=2Fe3++Mn2++2H2O��
��3������pHֵ�õ���������pH�ƣ������к���Fe3+��Al3+�����ӣ���ͼ�ɱ��Կ���������4.7���Խ�Fe3+��Al3+��ȥ��С��8.3�Ƿ�ֹMn2+Ҳ����������ֻҪ����pHֵ��4.7��8.3�伴�ɣ�
�ʴ�Ϊ��pH�ƣ�4.7��pH��8.3��
��4��ͼ�����Ӵ������£��뾶�м�С���ƣ���Ӧ�������ʼ�С������ʱ��ĵ������������ӵ������ʾ�������Fe3+��Al3+����������������������ʵͣ������к���Fe2+��Al3+��Ca2+��Pb2+���������ӣ��ɳ�����pH��Χ֪��Fe2+�ij�����Mn2+���ӵij��������pH�ӽ�����Fe3+������Զ���ʿ��Խ�Fe2+������Fe3+�����ӣ��������ʵ�ͼ���Կ�����Ca2+��Pb2+�������ʽϸߣ�
�ʴ�Ϊ������ʱ�䡢�������Ӱ뾶���������ӵ�ɣ�Pb2+��Ca2+��
��5��c mol•L-1CaCl2��Һ��2.00��10-2 mol•L-1Na2SO4��Һ�������ϣ���Ϻ�Na2SO4��Ũ��Ϊ1.00��10-2 mol•L-1������Ksp��CaSO4��=c��Ca2+��•c��SO42-������֪�����Һ��c��Ca2+��=$\frac{Ksp��CaS{O}_{4}��}{c��S{{O}_{4}}^{2-}��}$=$\frac{9.10��1{0}^{-6}}{1.00��1{0}^{-2}}$ mol•L-1=9.10��10-4 mol•L-1�����Ի��ǰc=2��9.10��10-4 mol•L-1=1.82��10 -3mol•L-1��
�ʴ�Ϊ��1.82��10 -3mol•L-1��
���� �������Ʊ������̵���������Ϊ֪ʶ���壬���黯ѧ��Ӧ����д���������е����⣬��Ŀ�Ѷ��еȣ�����ע��������ݴ���������ͼ�����������

| ʱ�䣨min�� | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| n��A�� | 2.00 | 1.90 | 1.82 | 1.76 | 1.64 | 1.54 | 1.50 | 1.50 | 1.50 |
| n��B�� | 1.00 | 0.95 | 0.91 | 0.88 | 0.82 | 0.77 | 0.75 | 0.75 | 0.75 |
| n��C�� | 0 | 0.10 | 0.18 | 0.24 | 0.36 | 0.46 | 0.50 | 0.50 | 0.50 |
�ش��������⣺
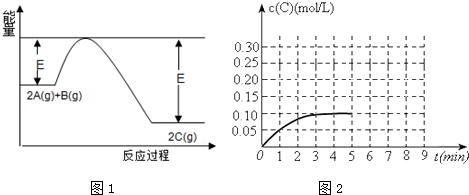
��1����Ӧ������Ӧ��H��������ڡ���С�ڡ���0��
��2���÷�Ӧ�ﵽƽ��ʱ������˵������ȷ����D��
��A��������ܶȲ��ٸı� ��B��A�����ʵ���Ũ�Ȳ��ٸı�
��C���ų������յ��������ٱ仯 ��D��v����A��=v����A��=0
��3��t��Cʱ����һ�ݻ�Ϊ2L�ĺ����ܱ������ڼ���0.4molA��0.6molB����һ�������·�����Ӧ����Ӧ��C�����ʵ���Ũ�ȱ仯�����2ͼ�����¶��£���Ӧ���е�1����ʱB���ʵ���Ϊ0.55mol��
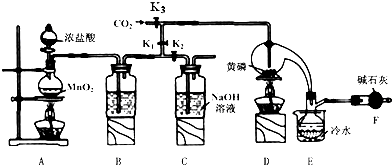
��֪����������Cl2��Ӧ����PCl3�������Cl2��Ӧ����PCl5��PCl3��ˮ��ǿ��ˮ������H3PO3��HCl����O2������POCl3��POCl3����PCl3��PCl3��POCl3���۷е������
| ���� | �۵�/�� | �е�/�� |
| PCl3 | -112 | 75.5 |
| POCl3 | 2 | 105.3 |
��1��B����װ�Լ���ŨH2SO4��E����ˮ������������PCl3��ֹ��ӷ���
��2��F�м�ʯ�ҵ����������ն������������ֹ�����е�ˮ����������ƿ�к�PCl3 ��Ӧ��
��3��ʵ��ʱ�����װ�������Ժ��ȴ�K3ͨ������CO2����Ѹ�ټ�����ף�ͨ����CO2���������ž�װ���еĿ�������ֹ������ȼ��
��4���ֲ�Ʒ�г�����POC13��PCl5�ȣ���������ȳ�ȥPCl5��ͨ��������ʵ��������ƣ������ɵõ��ϴ�����PCl3��
��5��ʵ�����ʱ����������C�е��Լ����ն����������C�з�Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+2H2O��
��6��ͨ�����淽���ɲⶨ��Ʒ��PCl3������������
��Ѹ�ٳ�ȡ1.00g��Ʒ����ˮ��Ӧ�����250mL��Һ��
��ȡ������Һ25.00mL�������м���10.00mL 0.1000mol/L��ˮ����ַ�Ӧ��
�����������Һ�м��뼸�ε�����Һ����0.1000mol/L��Na2S2O3����Һ�ζ���
���ظ��ڡ��۲�����ƽ������Na2S2O3��Һ8.40mL��
��֪��H3PO3+I2=H3PO4+2HI��I2+2Na2S2O3=2NaI+Na2S4O6��
�����������ݣ�����ⶨ������û��������Ӧ���ò�Ʒ��PCl3����������Ϊ79.75%��
| A�� | X��Y��Z�γɵĻ������в����ܼ������Ӽ� | |
| B�� | X��Z��M��Y��Z��W�����γ�ԭ�Ӹ�����Ϊ1��1�Ļ����� | |
| C�� | Y��Z��W��M�γɵļ����Ӱ뾶��С��ϵΪY��Z��M��W | |
| D�� | W��Cl�γɻ�������۵����M��Cl�γɻ�������۵� |
| A�� | ˮ�����c�� H+��=1��l0-13 mol/L����Һ�У�K+��Na+��SiO32-��SO42- | |
| B�� | ��ʹʯ�����ɫ����Һ�У�Na+��Fe3+��SO42-��Cl- | |
| C�� | $\frac{{K}_{W}}{c��O{H}^{-}��}$=l��l0-13mol/L����Һ�У�Ba2+��ClO-��Cl-��NO3- | |
| D�� | ��������Ӧ�ų�H2����Һ�У�Fe2+��K+��SO42-��Cl- |
| A�� | Na+��Mg2+��Br-��Ba2+ | B�� | Na+��SO32-��SO42-��K+ | ||
| C�� | K+��MnO4-��NO3-��Na+ | D�� | K+��Ca2+��SO32-��Cl- |
 ���÷���������Ҫ�ɷ�ΪAl��������Fe��Si�ȣ��ȿ���ȡ�л��ϳɴ���AlBr3�ֿ���ȡ��ˮ������������[A12��SO4��3•18H2O]��
���÷���������Ҫ�ɷ�ΪAl��������Fe��Si�ȣ��ȿ���ȡ�л��ϳɴ���AlBr3�ֿ���ȡ��ˮ������������[A12��SO4��3•18H2O]��