��Ŀ����
����Ŀ����̼�������������ã����������粣�����۾�Ƭ�ȡ�ij��̼����(M)�ĺϳ�·�����£�

��֪��
��.D�ķ���ʽΪC3H4O3���˴Ź�������ֻ��һ���
��.R1COOR2+R3OH![]() R1COOR3+ R2OH
R1COOR3+ R2OH
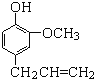
(1)A�������� _______��D�Ľṹ��ʽΪ_____��
(2)B��C�ķ�Ӧ����______��
(3)����H��˵����ȷ������_______��
A.����ʽΪC15H16O2
B.�������ԣ��DZ��ӵ�ͬϵ��
C.������̼ԭ�ӿ��ܹ���
D.1mol H��Ũ��ˮȡ�������л����������NaOH10mol
(4)д��A��B��ѧ����ʽ________��
(5)����ľ�̼��������H�����(![]() )�ۺϵõ�����д���þ�̼�����Ľṹ��ʽ________��
)�ۺϵõ�����д���þ�̼�����Ľṹ��ʽ________��
(6)H��ͬ���칹��������������������___�֣�
��������(![]() )�ṹ ���ܷ���ˮ���������Ӧ �������һ�����һ������
)�ṹ ���ܷ���ˮ���������Ӧ �������һ�����һ������
(7)F��G�������ϳ�
CH2=CHCH3![]() K
K![]() L
L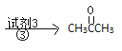
���Լ�1ΪHBr����L�Ľṹ��ʽΪ_______���۵ķ�Ӧ������_____��
���𰸡�1,2-�������� ![]() ȡ����Ӧ AD
ȡ����Ӧ AD 
 18
18 ![]() Cu/Ag��O2����
Cu/Ag��O2����
��������
��������ͼ��±����A(![]() )������������Һ��ˮ������B��BΪ
)������������Һ��ˮ������B��BΪ![]() ��C(
��C(![]() )���������������·�Ӧ����D��D��״���������������E(
)���������������·�Ӧ����D��D��״���������������E(![]() )��D�ķ���ʽΪC3H4O3���˴Ź�������ֻ��һ��壬���DΪ
)��D�ķ���ʽΪC3H4O3���˴Ź�������ֻ��һ��壬���DΪ![]() ���ݴ˷������(1)~(6)��
���ݴ˷������(1)~(6)��
(7)CH2=CHCH3��HBr�����ӳɷ�Ӧ����CH3CHBrCH3��CH3CHBrCH3ˮ������CH3CHOHCH3�����CH3CHOHCH3�������ɵõ���ͪ���ݴ˷������
(1)A�Ľṹ��ʽΪ![]() ������Ϊ1,2-�������飬D�Ľṹ��ʽΪ
������Ϊ1,2-�������飬D�Ľṹ��ʽΪ![]() ���ʴ�Ϊ��1,2-�������飻
���ʴ�Ϊ��1,2-�������飻![]() ��
��
(2)BΪ![]() ��CΪ
��CΪ![]() ��B��
��B��![]() ����ȡ����Ӧ���ɣ��ʴ�Ϊ��ȡ����Ӧ��
����ȡ����Ӧ���ɣ��ʴ�Ϊ��ȡ����Ӧ��
(3)HΪ ��A.����H�Ľṹ��ʽ��H�ķ���ʽΪC15H16O2����A��ȷ��B.H�к��з��ǻ������������ԣ�������2�������������ڱ��ӵ�ͬϵ���B����C.�ṹ�к���
��A.����H�Ľṹ��ʽ��H�ķ���ʽΪC15H16O2����A��ȷ��B.H�к��з��ǻ������������ԣ�������2�������������ڱ��ӵ�ͬϵ���B����C.�ṹ�к���![]() ����������ṹ����˷�����̼ԭ��һ�������棬��C����D.���ǻ�����λ�Ͷ�λ��ԭ���ܹ�����ȡ����Ӧ��1mol H��Ũ��ˮȡ�������л�������ຬ��4����ԭ�ӣ����ǻ�����ԭ�Ӿ�������NaOH������ԭ��ˮ�����ɵķ��ǻ�Ҳ�����������Ʒ�Ӧ�����1mol
����������ṹ����˷�����̼ԭ��һ�������棬��C����D.���ǻ�����λ�Ͷ�λ��ԭ���ܹ�����ȡ����Ӧ��1mol H��Ũ��ˮȡ�������л�������ຬ��4����ԭ�ӣ����ǻ�����ԭ�Ӿ�������NaOH������ԭ��ˮ�����ɵķ��ǻ�Ҳ�����������Ʒ�Ӧ�����1mol  �������10mol�������ƣ���D��ȷ���ʴ�Ϊ��AD��
�������10mol�������ƣ���D��ȷ���ʴ�Ϊ��AD��
(4)A��B��±������ˮ�ⷴӦ����Ӧ�Ļ�ѧ����ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(5)����ľ�̼��������H�����(![]() )�ۺϵõ���
)�ۺϵõ��� ��
��![]() �������۷�Ӧ����
�������۷�Ӧ���� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(6)H( )�IJ����Ͷ�=8��H��ͬ���칹��������������������������(
)�IJ����Ͷ�=8��H��ͬ���칹��������������������������(![]() )�ṹ�������Ͷ�=7�����ܷ���ˮ���������Ӧ��˵���ṹ�к���������ȩ����������ڼ����������ʣ������IJ����Ͷ�=1����������Ϊ���ͽṹ���������һ�����һ�����ϣ������һ���һ��������
)�ṹ�������Ͷ�=7�����ܷ���ˮ���������Ӧ��˵���ṹ�к���������ȩ����������ڼ����������ʣ������IJ����Ͷ�=1����������Ϊ���ͽṹ���������һ�����һ�����ϣ������һ���һ��������![]() ��
��![]() ��
��![]() ��
��![]() 4�������HCOO-��λ�÷ֱ�Ϊ6��6��3��3����18�ֽṹ���ʴ�Ϊ��18��
4�������HCOO-��λ�÷ֱ�Ϊ6��6��3��3����18�ֽṹ���ʴ�Ϊ��18��
(7)CH2=CHCH3��HBr�����ӳɷ�Ӧ����CH3CHBrCH3��CH3CHBrCH3ˮ������CH3CHOHCH3�����CH3CHOHCH3�������ɵõ���ͪ�����LΪCH3CHOHCH3����Ӧ�۵�����ΪCu/Ag��O2�������ʴ�Ϊ��CH3CHOHCH3��Cu/Ag��O2������