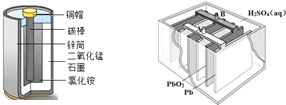
��Ŀ����
����Ŀ����ͼΪԪ�����ڱ���һ���֣������NԪ�ص�λ�ã��ش��������⣺
�� | N | |||||||
�� | �� | �� | �� |
��ش��������⣺
(1)д����4���ڡ����ͬ��Ԫ�ص����ƣ�___________��
(2)�ٵĵ��ʱ���Ϊ��21���͵���Դ�������������������غʹ������������⻯�������ˮ��Ӧ�������������⻯�����ʽΪ___________��д�����⻯����ˮ��Ӧ�Ļ�ѧ����ʽ_____________________��
(3)��(Ga)���ͬ���壬д���ص���������NaOH��Ӧ�Ļ�ѧ����ʽΪ____________��
(4)��Ԫ�صķǽ����ԱȢ�______(����ǿ����������)���о�һ����ѧ��ʵ����˵��_______________________________________________��
(5)NԪ�ش���N2��N4��ͬ�������塣��֪N4Ϊ��������ṹ��N��N����Ϊ167 kJ��mol-1��N��N����Ϊ942 kJ��mol-1��д��N4(g)ת��ΪN2(g)���Ȼ�ѧ����ʽ��______________________________________��
���𰸡�� ![]() LiH+H2O ��H2��+ LiOH Ga2O3 + 2OH- �� 2GaO2-+H2O �� ���ԣ�H2SO4��HClO4(���ȶ��ԣ�H2S��HCl�ȣ���������) N4(g)�� 2N2(g) ��H=-882 kJ��mol-1
LiH+H2O ��H2��+ LiOH Ga2O3 + 2OH- �� 2GaO2-+H2O �� ���ԣ�H2SO4��HClO4(���ȶ��ԣ�H2S��HCl�ȣ���������) N4(g)�� 2N2(g) ��H=-882 kJ��mol-1
��������
����Ԫ�������ڱ��е�λ�ÿ��жϸ�Ԫ��Ϊ��Li��Al��S��Cl��Ar
��1����4�������Arͬ��Ԫ�ص����ƣ�봣�
��2���⻯﮿�����ˮ��Ӧ�������������⻯�����ʽΪ![]() ��
��
��3��ģ���������������ķ�Ӧ��д��Ga2O3 �� OH- ��Ӧ����GaO2-�����ӷ���ʽ��
��4��ͬһ���ڴ����ҷǽ���������ǿ���ɴ����������ˮ��������ǿ������H2���ϵ����Ѽ������⻯���ȶ��Եȶ��Ƚϡ�
��5���÷�Ӧ�ʱ�=��Ӧ�����-��������ܣ��ݴ���д�Ȼ�ѧ����ʽ��
��1����4�������Arͬ��Ԫ�ص����ƣ�봣�
��2���⻯﮵���ʽΪ![]() ���⻯﮿�����ˮ��Ӧ����������������ﮣ���ѧ����ʽ��LiH+H2O��H2��+ LiOH ��
���⻯﮿�����ˮ��Ӧ����������������ﮣ���ѧ����ʽ��LiH+H2O��H2��+ LiOH ��
��3��ģ���������������ķ�Ӧ��д��Ga2O3 �� OH- ��Ӧ����GaO2-�����ӷ���ʽ��Ga2O3 + 2OH- �� 2GaO2-+H2O��
��4��ͬһ���ڴ����ҷǽ���������ǿ��S�ǽ�����С��Cl�����ԣ�H2SO4��HClO4���ȶ��ԣ�H2S��HCl�Ⱦ���˵����
��5���÷�Ӧ�ʱ�=��Ӧ�����-���������=6��167kJ��mol��1-2��942kJ��mol��1=-882kJ��mol��1����÷�Ӧ�Ȼ�ѧ����ʽΪN4��g��=2N2��g����H=-882 kJ��mol��1��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���ϳɰ��ķ�ӦΪN2(g)��3H2(g)![]() 2NH3(g)���±���ij�η�ӦʱNH3��Ũ�ȱ仯��
2NH3(g)���±���ij�η�ӦʱNH3��Ũ�ȱ仯��
ʱ��(min) | 0 | 5 | 10 | 15 | 20 | 25 |
c(NH3)(mol��L-1) | 0 | 0.30 | 0.44 | 0.50 | 0.50 | 0.53 |
����˵����ȷ����
A. 0��10 minʱ����H2��ʾ��ƽ����Ӧ����Ϊ0.044mol��L-1��min-1
B. ����ѹǿ�ͽ����¶Ⱦ��ܼӿ�ϳɰ��ķ�Ӧ����
C. ��ͼ����֪���÷�Ӧ��15��20 minʱ���ڻ�ѧƽ��״̬
D. ��Ӧ������������ܶȱ��ֲ��䣬˵���÷�Ӧһ���ﵽƽ��״̬