��Ŀ����
��14�֣�ij��ȤС���ѧ������Mg��CO2��Ӧԭ�����Ʋ���ҲӦ����CO2��ȼ�գ�Ϊ��ȷ�������ɲ��ﲢ����ʵ����֤��ijͬѧ�������ͼ��ʾװ�ý���ʵ�飨��֪PdCl2�ܱ�CO��ԭ�õ���ɫ��Pd������ش��������⣺
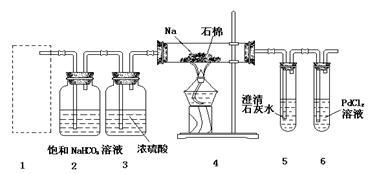
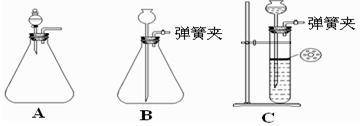
��1��Ϊʹ��Ӧ�濪���ã������ͣ��������Ӧѡ����ͼ��ʾ װ�ã�����ĸ���ţ���
��2��ʵ������ȡ������̼�������õ�ҩƷ�� �����ţ���
��ʯ��ʯ���ڴ����С�մ�18.4 mol��L��1���ᣬ��11.2mol��L��1���ᣬ������ˮ����ľ̿�ۡ�
��3����2װ���ڽ��з�Ӧ�����ӷ���ʽΪ �����װ�������Բ�װ��ҩƷ��ȼ�ƾ���֮ǰ��װ�� �������ֱ�ţ��г��� ����ʱ���ٵ�ȼ�ƾ��ơ�
��4������װ��6���к�ɫ������װ��4�в������壨ֻ��һ�����ʣ������������ʹ����ʯ��ˮ����ǵ�����ų��������������̼��Ӧ�Ļ�ѧ����ʽΪ ��
����װ��6����Һ����������װ��4�в������壨���������ʣ������������ʹ����ʯ��ˮ����ǵ�����ų��������������̼��Ӧ�Ļ�ѧ����ʽΪ ��

��1��Ϊʹ��Ӧ�濪���ã������ͣ��������Ӧѡ����ͼ��ʾ װ�ã�����ĸ���ţ���

��2��ʵ������ȡ������̼�������õ�ҩƷ�� �����ţ���
��ʯ��ʯ���ڴ����С�մ�18.4 mol��L��1���ᣬ��11.2mol��L��1���ᣬ������ˮ����ľ̿�ۡ�
��3����2װ���ڽ��з�Ӧ�����ӷ���ʽΪ �����װ�������Բ�װ��ҩƷ��ȼ�ƾ���֮ǰ��װ�� �������ֱ�ţ��г��� ����ʱ���ٵ�ȼ�ƾ��ơ�
��4������װ��6���к�ɫ������װ��4�в������壨ֻ��һ�����ʣ������������ʹ����ʯ��ˮ����ǵ�����ų��������������̼��Ӧ�Ļ�ѧ����ʽΪ ��
����װ��6����Һ����������װ��4�в������壨���������ʣ������������ʹ����ʯ��ˮ����ǵ�����ų��������������̼��Ӧ�Ļ�ѧ����ʽΪ ��
��1��C(2��)����2���٢ݢ�(2��)��
��3��HCO3-+H+ =CO2��+ H2O(2��)��5(1��)����ɫ����(1��)��
=CO2��+ H2O(2��)��5(1��)����ɫ����(1��)��
��4��2CO2+2Na= Na2CO3+CO(3��)��3CO2+4Na= 2Na2CO3+C
������д�� �����ȡ���ȼ������ȷ��(3��),
�����ȡ���ȼ������ȷ��(3��),
��3��HCO3-+H+
 =CO2��+ H2O(2��)��5(1��)����ɫ����(1��)��
=CO2��+ H2O(2��)��5(1��)����ɫ����(1��)����4��2CO2+2Na= Na2CO3+CO(3��)��3CO2+4Na= 2Na2CO3+C
�������
 �����ȡ���ȼ������ȷ��(3��),
�����ȡ���ȼ������ȷ��(3��),��

��ϰ��ϵ�д�
�����Ŀ
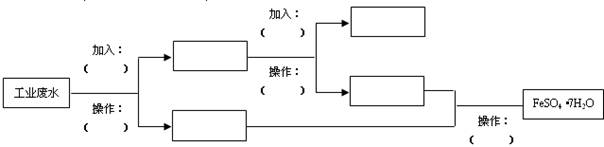
 ��OH
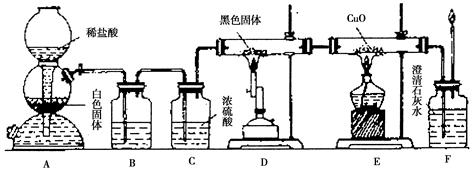
��OH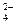 �������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������ǿ�����CO2��Ӱ�죩��
�������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������ǿ�����CO2��Ӱ�죩�� L-1H2SO4��0.01mol
L-1H2SO4��0.01mol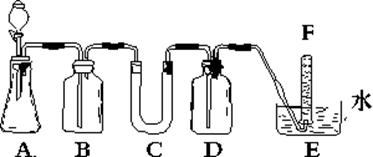
 ��
��
 CO��g��+H2O��g���ġ�H<O
CO��g��+H2O��g���ġ�H<O