��Ŀ����
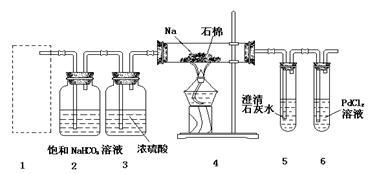
��7�֣�ijУѧ������С���ͬѧ�����ͼ��ʾʵ��װ�ã�������֤һ����̼���л�ԭ�ԡ��ش��������⡣
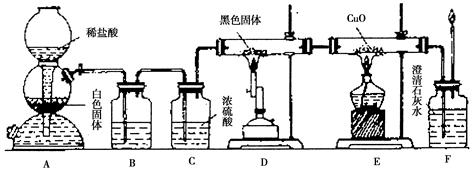
��1��װ��B�������˵��Լ��� ��
��2��װ��C�������� ��
��3��װ��D�к�ɫ����Ϊ̼���䷴Ӧ�Ļ�ѧ����ʽΪ ��
��4������Eװ���е�����֤��CO���л�ԭ�ԣ��йط�Ӧ�Ļ�ѧ����ʽ��
 ��
��
��5����Ҫ����װ��F�г��ֵ�����ȷ��һ����̼���л�ԭ�ԣ�Ӧ����ͼװ��D��E֮��������ͼ�е� װ�ã�����ţ���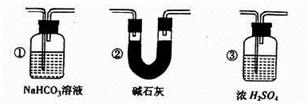

��1��װ��B�������˵��Լ��� ��
��2��װ��C�������� ��
��3��װ��D�к�ɫ����Ϊ̼���䷴Ӧ�Ļ�ѧ����ʽΪ ��
��4������Eװ���е�����֤��CO���л�ԭ�ԣ��йط�Ӧ�Ļ�ѧ����ʽ��
 ��
����5����Ҫ����װ��F�г��ֵ�����ȷ��һ����̼���л�ԭ�ԣ�Ӧ����ͼװ��D��E֮��������ͼ�е� װ�ã�����ţ���

��1������̼��������Һ��2�֣���
��2������CO2��1�֣�
��3��CO2 +C=2CO����1�֣�
��4��CO+Cu O="Cu+" CO2����1�֣���5���ڣ���2�֣�
O="Cu+" CO2����1�֣���5���ڣ���2�֣�
��2������CO2��1�֣�
|
|
 O="Cu+" CO2����1�֣���5���ڣ���2�֣�
O="Cu+" CO2����1�֣���5���ڣ���2�֣���

��ϰ��ϵ�д�
�����Ŀ
|
����ʵ���������������ȷ���ǣ� ��  �ٽ�NaNO3��KCl�Ļ��Һ���Ȳ�Ũ�����о������������ȹ���ʱ���ɷ����NaCl���� �ں����е�Ԫ�صķ��뼰����ʱ����Ҫ���ҵĽ�ȡҺ�м�������ϡ�������������Һ ��ֽ���������������Ӻ�ͭ����ʵ���У����������ֽ�����ɺ��ܽ������չ������ �ܾ���������������ˮ������������Na2CO3��Һ��FeSO4��Һ����ʹ������Һ���� ���ü��ȷ����Է��������غ͵ⵥ�ʵĻ�����Ϊ�ⵥ������������ ������һ�����ʵ���Ũ�ȵ���Һʱ������ȡҺ̬���ʵ���Ͳ��ˮϴ�ӣ�ϴ��Һ��������ƿ �߽�������ˮ�ε����۵⻯����ֽ�ϳ�����ͼ����˵����Ũ����ˮ�ܽ�I-������I2����Ũ����ˮ�ܽ�I2��һ�������ɵ�Ļ�����  �ཫ����CO2ͨ��Ca(ClO)2��Һ�ó�����Һ��˵��H2CO3�����Ա�HClO�� 
|
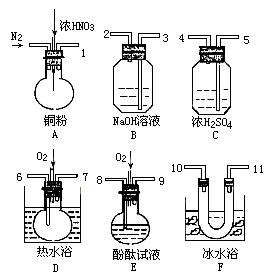
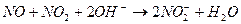 �� ����Һ���¶ȣ�NO2 21�棬 NO -152��
�� ����Һ���¶ȣ�NO2 21�棬 NO -152��