��Ŀ����
����Ŀ��ijͬѧ���к͵ζ����ⶨij�ռ���Һ��Ũ�ȡ���.ʵ�鲽�裺
(1)��________ (��������A������B��)ȡ�����ռ���Һ 25.00mL ����ƿ�У��μ� 2 �η�̪��ʾ����

(2)��¼ʢװ 0.1000mol/L �������Һ����ʽ�ζ��ܵij�ʼ���������ijʱ��Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ________mL��
![]()
(3)�ζ����ζ������У��۾�Ӧʼ��ע��________��
(4)�ζ��յ�������ǣ�________��
��.���ݼ�¼��

��.���ݴ���
(1)ƽ�����ĵ������Һ����� V =_____________mL��
(2)������ռ���Һ��Ũ�� c(NaOH) =_____________mol/L(��ȷ��С����� 4 λ)��
IV.������²����ᵼ�²ⶨ���ƫ�ߵ���_____________��
A.δ���������Һ��ϴ�ζ���
B.װ����Һǰ����ƿ������������ˮ
C.�ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ D.�۲����ʱ���ζ�ǰ���ӣ��ζ�����
���𰸡�A 0.70 ע����ƿ����Һ��ɫ�ı仯 ��Һ��ɫ��ȥ�����Ұ�����ڲ���ԭ 18.00 0.0720 AC
��������
��.ʵ�鲽�裺(1)������ҺΪNaOH��Һ���Լ��ԣ�AΪ��ʽ�ζ��ܣ�BΪ��ʽ�ζ��ܣ�
(2) ���ݵζ��ܵĽṹ�뾫ȷ����������
(3) �ζ�ʱ������ע����ƿ����Һ��ɫ�ı仯��
(4) ����Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��.���ݴ���
(1)��4��ʵ���������ƫ����ȡǰ�������ĵ������������ƽ��ֵ��
(2) ����c(NaOH) =c(����)��V(����)��V(NaOH)���㣻
IV. ����c(NaOH) =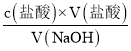 ��������������V(��)��Ӱ�죬�Դ��ж�Ũ�ȵ���
��������������V(��)��Ӱ�죬�Դ��ж�Ũ�ȵ���
��.ʵ�鲽�裺(1) ������ҺΪNaOH��Һ���Լ��ԣ�Ӧѡ���ʽ�ζ��ܣ���ѡ������A��
(2) ����ͼʾ��֪���ζ���Һ��Ķ���0.70mL��
(3) �ζ�ʱ�����ֿ��Ƶζ��ܵĻ��������ֲ�ͣ����ͬһ����ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯��
(4)��ƿ��Ϊ������Һ�ӷ�̪Ϊ��ɫ�����ﵽ�ζ��յ�ʱ����Һ��ɫ����ȥ�����Ұ�����ڲ���ԭ��
��.���ݴ���
(1) ��4��ʵ���������ƫ��ȡǰ�������ĵ������������ƽ�����ĵ������Һ����� V =![]() mL=18.00mL��
mL=18.00mL��
(2) c(NaOH) =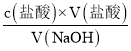 =
=![]() =0.0720mol/L��
=0.0720mol/L��
IV. A��δ���������Һ��ϴ�ζ��ܣ���Һ��Ũ�Ƚ��ͣ����ı�Һ�����ƫ����ⶨŨ��ƫ�ߣ���A��ȷ��
B��װ����Һǰ����ƿ������������ˮ����Ӱ�����ı�Һ��������Բⶨ���û��Ӱ�죬��B����
C���ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ�����ı�Һ���������ƫ����ⶨŨ��ƫ�ߣ���C��ȷ��
D���۲����ʱ���ζ�ǰ���ӣ��ζ����ӣ����ı�Һ���������ƫС����ⶨŨ��ƫ�ͣ���D����
�ʴ�ΪAC��