��Ŀ����
����Ŀ�������£���һԪ��HA����Һ��KOH��Һ�������ϣ���������仯����ʵ���������±���
ʵ���� | ��ʼŨ��/��mol��L��1�� | ��Ӧ����Һ��pH | |
c��HA�� | c(KOH) | ||
�� | 0.1 | 0.1 | 9 |
�� | x | 0.2 | 7 |
�����жϲ���ȷ����
A��ʵ������Ӧ�����Һ�У�c(K��)>c(A��)>c(OH��)>c(H��)
B��ʵ������Ӧ�����Һ�У�c(OH��)=c(K��)��c(A��)=![]() mol/L
mol/L
C��ʵ������Ӧ�����Һ�У�c(A��)��c(HA)>0.1mol��L��1
D��ʵ������Ӧ�����Һ�У�c(K��)=c(A��)>c(OH��) =c(H��)
���𰸡�B
��������
A�������ǵ������Ũ�Ȼ�ϣ�Ӧ��ǡ����ȫ��Ӧ��������KA����Ӧ����Һ�Լ��ԣ�˵��HA�����ᣬA������ˮ�⣬A����H2O![]() HA��OH��������Ũ�ȴ�С˳����c(K��)>c(A��)>c(OH��)>c(H��)����A˵����ȷ��B�����ݵ���غ㣬��c(K��)��c(H��)=c(OH��)��c(A��)������c(OH��)=c(K��)��c(H��)��c(A��)=Kw/10��9mol��L��1����B˵������C����ΪHA�����ᣬ��Ӧ����Һ�����ԣ�����ΪHA��KA�����HA��Ũ��Ӧ����0.2mol�����������غ㣬c(A��)��c(HA)>0.2/2mol��L��1=0.1mol��L��1����C˵����ȷ��D�����ݵ����ԣ�c(K��)��c(H��)=c(OH��)��c(A��)����Һ�����ԣ���c(H��)=c(OH��)��c(K��)=c(A��)������Ũ�ȴ�С˳����c(K��)=c(A��)> c(H��)=c(OH��)����D˵����ȷ��
HA��OH��������Ũ�ȴ�С˳����c(K��)>c(A��)>c(OH��)>c(H��)����A˵����ȷ��B�����ݵ���غ㣬��c(K��)��c(H��)=c(OH��)��c(A��)������c(OH��)=c(K��)��c(H��)��c(A��)=Kw/10��9mol��L��1����B˵������C����ΪHA�����ᣬ��Ӧ����Һ�����ԣ�����ΪHA��KA�����HA��Ũ��Ӧ����0.2mol�����������غ㣬c(A��)��c(HA)>0.2/2mol��L��1=0.1mol��L��1����C˵����ȷ��D�����ݵ����ԣ�c(K��)��c(H��)=c(OH��)��c(A��)����Һ�����ԣ���c(H��)=c(OH��)��c(K��)=c(A��)������Ũ�ȴ�С˳����c(K��)=c(A��)> c(H��)=c(OH��)����D˵����ȷ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������Ҫ��������и�С�⣺
��.��1��������ϡ��ǿ�ᡢǿ�Ӧ����1mol H2O��l��ʱ�ų�57.3kJ��������д����ʾϡ�����ϡ����������Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ________ ��
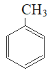
��2����֪���ұ��������Ʊ���ϩ��Ӧ�����ڼ������ʱ���˴��ɿ���Ϊ![]() ��
�� ![]()
![]()
![]() +H2��g��
+H2��g��
��ѧ�� | C��H | C��C | C=C | H��H |
����/kJ��mol��1 | 412 | 348 | 612 | 436 |
����������Ӧ����H��________ kJ��mol��1��
��.25 ��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ����ش��������⣺
��ѧʽ | CH3COOH | H2CO3 | HClO |
����ƽ�ⳣ�� | 1.7��10��5 | K1��4.3��10��7 K2��5.6��10��11 | 3.0��10��8 |
��1��CH3COOH��H2CO3��HClO��������ǿ������˳��Ϊ_______��
��2��������CO2����ͨ��NaClO��Һ�У�д����Ӧ�����ӷ���ʽ��________��