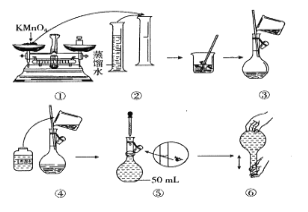
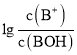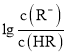��Ŀ����
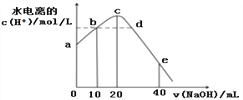
����Ŀ��ij��ѧС���������ͼ��ʾ�����ֻ�ʵ��װ�ã��о������£���1L0.1mol/L H2A��Һ����μ����Ũ��NaOH��Һʱ��pH�仯����������Ƴ���Һ�к�AԪ�ص��������ʵ�����������ҺpH�Ĺ�ϵ��ͼ��ʾ��������˵��������ȷ���ǣ� ��
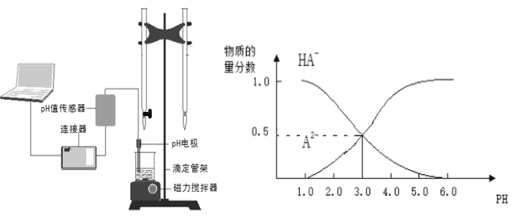
A��pH=4.0ʱ��ͼ��n(HA��)ԼΪ0.0091mol
B����ʵ��Ӧ����ߵ���ʽ�ζ��ܻ����ұ�ʽ�ζ��ܲ��ӷ�̪��ָʾ��
C�������£������ʵ���Ũ�ȵ�NaHA��Na2A��Һ�������Ϻ���ҺpH=3.0
D��0.1mol/LNaHA��Һ�д���c(A2��)��c(HA��)��c(H2A)��0.1mol/L
���𰸡�A
��������
���������A��pH=4.0ʱ�������Һ���δ֪��������n(HA��)����A����B����ͼ���֪����ʵ�������к��������������������Һ������Ӧ����ߵ���ʽ�ζ��ܻ����ұ�ʽ�ζ��ܲ��ӷ�̪��ָʾ������B��ȷ��C����ͼ���֪�������£������ʵ���Ũ�ȵ�NaHA��Na2A��Һ�������Ϻ���ҺpH=3.0����C��ȷ��D�����������غ��֪��0.1mol/LNaHA��Һ�д���c(A2��)��c(HA��)��c(H2A)��0.1mol/L����D��ȷ��

����Ŀ���ⶨ0.1 mol��L-1 Na2SO3��Һ�������ٽ��¹����е�pH���������¡�
ʱ�� | �� | �� | �� | �� |
�¶�/�� | 25 | 30 | 40 | 25 |
pH | 9.66 | 9.52 | 9.37 | 9.25 |
ʵ������У�ȡ�٢�ʱ�̵���Һ�����������ữ��BaCl2��Һ���Ա�ʵ�飬�ܲ�����ɫ�����ࡣ
����˵������ȷ����
A. Na2SO3��Һ�д���ˮ��ƽ����![]() +H2O
+H2O![]()
![]() +OH
+OH
B. ����pH������ͬ��������![]() Ũ�ȼ�С��ɵ�
Ũ�ȼ�С��ɵ�
C. �١����Ĺ��������¶Ⱥ�Ũ�ȶ�ˮ��ƽ���ƶ������Ӱ��һ��
D. ��������Kwֵ���
����Ŀ���±��г��ˢ١���ʮ��Ԫ�������ڱ��е�λ�á�
�� ���� | ��A | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | �� | ||
�ش��������⣺
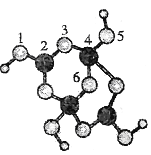
(1)�١��ܰ�ԭ�Ӹ�����1:1 ��ɵķ��ӵĵ���ʽΪ____________________ ���ɢڡ�������Ԫ����ɵ�һ����������ĽṹʽΪ _____________________��
(2)��10��Ԫ���У���ѧ��������õ�Ԫ����_____________(��Ԫ�ط��ţ���ͬ)���õ���������ǿ��ԭ����__________________��ʧ����������ǿ�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ��_________________________��
(3)�û�ѧ����ʽ��ʾ�ں͢�����Ԫ�صķǽ�����ǿ����________________________ ��
(4)Ԫ�آ۵���̬�⻯���Ԫ�آ����̬�⻯���У������Ʊ����� ____________________(�ѧʽ)
(5)Ԫ�آݵ�����������Ӧ��ˮ������Ԫ�آߵ�����������Ӧ��ˮ���ﷴӦ�������ӷ���ʽΪ ______________________________��
(6)Ԫ�� �١��ܡ�������֮������γ��������͵Ļ����д��һ�ֹ��ۻ�����Ļ�ѧʽ��___________________ ��д��һ�����ӻ�����Ļ�ѧʽ��______________________��
(7)д���ĵ����û����ڵĵ��ʵĻ�ѧ����ʽ��________________________��