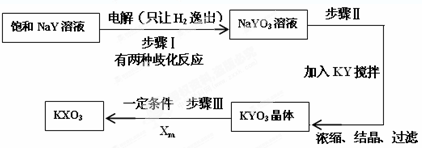
��Ŀ����
8�� һ���º��ݵ������ɸ���ֳ�A��B���ң���������ɻ����������¶�ʼ�ձ���20�森A����װ��3mol H2��1mol O2�Ļ�����壬��ʱ����λ����ͼ��ʾ����ش��������⣺
һ���º��ݵ������ɸ���ֳ�A��B���ң���������ɻ����������¶�ʼ�ձ���20�森A����װ��3mol H2��1mol O2�Ļ�����壬��ʱ����λ����ͼ��ʾ����ش��������⣺��1��B���л����������ʵ���Ϊ2mol��
��2����֪B���л��������ܶ���ͬ��ͬѹ�º����ܶȵ�9.125������B����H2��Cl2�����ʵ���֮��Ϊ1��1��
��3��һ�������£�ʹA��B�����ڵ�����ֱ��ַ�Ӧ���ٻָ���20�棬������λ���ڿ̶�2�������������������ѹǿ�뷴Ӧǰ����ѹǿ�ı�Ϊ1��2
��4������3������HCl������500mL NaOH��Һǡ����ȫ���գ���NaOH��Һ�����ʵ���Ũ��Ϊ4mol•L-1��
���� ��1��ͬ��ͬѹ�£���������ʵ���֮�ȵ��������֮�ȣ�
��2���������ƽ����Է�������Ϊ9.125��4=36.5���൱��HCl����Է���������
��3�����ݷ���ʽ���㷴Ӧ��������ʣ���������ʵ������ָ�ԭ�¶Ⱥ�����������ѹǿ��ȣ����֮�ȵ��������ʵ���֮�ȣ�����ȷ������ͣ����λ�ã�
B���п������ʵ������䡢�¶Ȳ��䣬��Ӧǰ��ѹǿ֮���뷴Ӧǰ������ɷ��ȣ�
��4����HCl+NaOH=NaCl+H2O����֪n��NaOH��=n��HCl�����ٸ���c=$\frac{n}{V}$���㣮
��� �⣺��1��A��B����ѹǿ���¶���ͬ����������ʵ���֮�ȵ���������֮�ȣ���B�����������ʵ���Ϊ4mol��$\frac{2}{4}$=2mol���ʴ�Ϊ��2��
��2���������ƽ����Է�������Ϊ9.125��4=36.5���൱��HCl����Է�����������B����H2��Cl2�����ʵ���֮��Ϊ1��1���ʴ�Ϊ��1��1��
��3����2H2+O2$\frac{\underline{\;��ȼ\;}}{\;}$2H2O����֪������ʣ�࣬ʣ������Ϊ3mol-2mol=1mol��B���з���H2+Cl2=2HCl����������ʵ���Ϊ2mol���ָ�ԭ�¶Ⱥ�����������ѹǿ��ȣ����֮�ȵ��������ʵ���֮�ȣ���A��B���ҵ����֮��Ϊ1mol��2mol=1��2�������ͣ����2�̶ȴ���
B���п������ʵ������䡢�¶Ȳ��䣬��Ӧǰ��ѹǿ֮���뷴Ӧǰ������ɷ��ȣ�������������ѹǿ�뷴Ӧǰ����ѹǿ֮��Ϊ2��4=1��2��
�ʴ�Ϊ��2��1��2��
��4����HCl+NaOH=NaCl+H2O����֪n��NaOH��=n��HCl��=2mol����NaOH��Һ�����ʵ���Ũ��Ϊ$\frac{2mol}{0.5L}$=4mol/L���ʴ�Ϊ��4��
���� ���⿼�鰢��٤�����ɼ������ۣ����ؿ���ѧ������������ע�����PV=nRT���Ⱒ��٤�����ɼ������ۣ�

| A�� | ��ˮ���ƾ� | B�� | ��ˮ������ | C�� | ��ˮ�����Ȼ�̼ | D�� | ��ˮ�����Ȼ�̼ |
| A�� | 2.12g | B�� | 3.51g | C�� | 4.22g | D�� | 5.28g |
| A�� | ��ҵ�Ϻϳɰ���ԭ�� | |
| B�� | ��ҵ�ϲ������� | |
| C�� | �ȵĴ�����Һϴ��Ч������ | |
| D�� | ����ʱ���Թܵײ���NH4Cl����ת�����Թ��϶� |
| ʵ������ | |
| 1�����ĥ������Ƭ�м���ϡ���� | |
| 2�����ĥ������Ƭ�м���Na0H��Һ |
���ӷ���ʽ����2Al+6H+=2Al3++3H2������2Al+2OH-+2H2O=2AlO2-+3H2����
| A�� | ����ˮ��ͨ�����������ˮ��Ư������ǿ | |
| B�� | ��ˮ�м���NaCl���壬����Һ��pH��Ӱ�� | |
| C�� | �����£�pH=2����ˮ�У�c ��Cl-��+c �� ClO-��+c ��OH -��=0.01mol•L-1 | |
| D�� | ����ˮ�еμ�����NaHCO3��Һ�����ӷ�Ӧ����ʽΪ��Cl2+2HCO3-�T2CO2��+Cl -+ClO -+H2O |
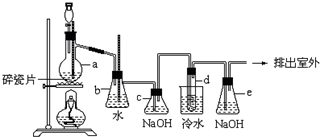 1��2-��������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ���2.18g/cm3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ���п�������ͼ��ʾװ���Ʊ�1��2-�������飮���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Ũ��ˮ�����渲������ˮ����
1��2-��������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ���2.18g/cm3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ���п�������ͼ��ʾװ���Ʊ�1��2-�������飮���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Ũ��ˮ�����渲������ˮ����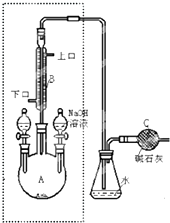 �Ʊ��屽��ʵ��װ����ͼ��ʾ���ش��������⣺
�Ʊ��屽��ʵ��װ����ͼ��ʾ���ش��������⣺ ������Ϊ��װ���п��Լ��뱽��CCl4Һ�壮
������Ϊ��װ���п��Լ��뱽��CCl4Һ�壮 ��
��