��Ŀ����
ij��ɫ����Һ�п��ܺ��� ��
��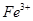 ��
�� ��
�� ��
��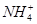 �������ӡ�ijͬѧ��������ʵ�飺
�������ӡ�ijͬѧ��������ʵ�飺
I�����������ϡ���ᣬ�а�ɫ�������ɡ�
II�����ˣ�ȡ������Һ�������м��������ϡ���ᣬ���а�ɫ�������ɡ�
III����ȡ��������II�е���Һ������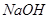 ��Һ����Һ�ʼ��ԣ����ȣ��ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣
��Һ����Һ�ʼ��ԣ����ȣ��ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣
��ش��������⣺
��1������Һ��һ�����е�������__________��һ�������е�������___________��
��2������һ�����Ӳ���ȷ���Ƿ���ڣ������������ӵ�ʵ�鷽����__________���۲쵽��������_______________________��
��3������д��������III���в�����������ӷ���ʽ__________________��
��8�֣�
��1�� ��
�� ��
��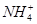 ��3�֣�
��3�֣� 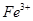
��2����ɫ��Ӧ ����ɫ�ܲ����۲쵽��ɫ����
��3��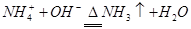 ��2�֣�
��2�֣�
�������������ij��ɫ����Һ˵����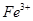 �� I˵����AgCl������һ������
�� I˵����AgCl������һ������ .II�����ᱵ������˵������
.II�����ᱵ������˵������ ��III���ȣ��ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣˵���ǰ���һ����笠����ӡ�
��III���ȣ��ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣˵���ǰ���һ����笠����ӡ�
���㣺���������ӵķ�Ӧ�Լ������IJ�����ȷ�����ӵ����࣬������ص�ʵ�����⡣

 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д��������ӷ���ʽ��д��ȷ����
| A��̼������ڴ��CaCO3+2H+��Ca2++CO2��+ H2O |
| B����״���½�112ml����ͨ��6ml 1mol/L�ĵ⻯������Һ��3Cl2+2Fe2++4I-��6Cl-+2Fe3++2I2 |
| C��Ư����Һ��ͨ������SO2��Ca2++2ClO-+SO2+H2O��CaSO3��+ 2HClO |
| D�������ʯ��ˮ�м������̼��������ҺCa2++2OH-+2HCO3-��CaCO3��+ 2H2O+CO32- |
��Һ����ˮ���������c(OH��)��1��10��12mol/L���������������Һ��һ���ܴ����������������
| A��Fe2+ Na+ NO3�� Cl�� | B��K+Na+ Cl��AlO2�� |
| C��K+ Ba2+ Cl�� NO3�� | D��K+ NH4+ SO42�� CO32�� |
ij��ɫ��Һ�к���K����Cl����OH����SO32-��SO42-��Ϊ�˼����OH������������������ӣ��������ᡢ���ᡢ��������Һ������������Һ����ˮ�ͷ�̪�����Լ����������ʵ�鲽�裬����¼������� �����йؽ����������� �� ��
�����йؽ����������� �� ��
| A���Լ�����AgNO3��Һ���Լ�����HNO3������1�а�ɫ������AgCl |
| B������3�а�ɫ������BaSO4 |
| C���Լ��������ᣬ�Լ��������� |
| D����������2�����ӷ���ʽ�ǣ�Br2��2H2O��SO2=4H����2Br����SO42- |
���и�����������Һ���ܴ����������
| A��Cu2+��Mg2+��SO42-��NO3- | B��H+��Mg2+��SO42-��ClO- |
| C��Ag+��Na+��NO3-��Cl- | D��NH4+��Ba2+��NO3-��OH- |
�������ӷ���ʽ����д��ȷ���ǣ� ��
| A������ϡ���ᷴӦ��2Fe��6H��=2Fe3����3H2�� |
| B��̼��ƺ�ϡ���ᷴӦ��CaCO3��2H��=Ca2����CO2����H2O |
| C����Cl2ͨ��KI��Һ�У�I����Cl2=Cl����I2 |
| D������þ��Һ������������Һ��Ӧ��Mg2����2OH��=Mg(OH)2�� |
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����
| A��NO2ͨ��ˮ�У�3NO2+H2O=2H++2NO��3+NO |
B��������ͭ�缫���CuSO4��Һ��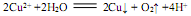 |
| C��SO2ͨ��FeCl3��Һ�У�SO2+Fe3++2H2O=SO2��4+Fe2++4H+ |
D��������CO2ͨ��NaAlO2��Һ�У� |
�����£����и���������ָ����Һ��һ���ܴ���������ǣ� ��
| A��1.0 mol��L��1��CaCl2��Һ��Na����K����Cl����CO32- |
| B��1.0 mol��L��1��HNO3��Һ��K����[Ag(NH3)2]����Cl����SO42- |
| C��1.0 mol��L��1��KI��Һ��Na����Fe3����Cl����SO42- |
| D������������Һ��Cu2����SO42-��Mg2����Cl�� |