��Ŀ����
��10�֣�
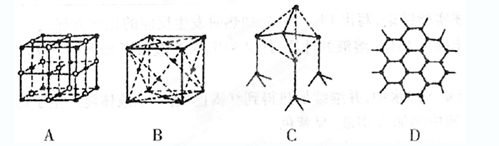
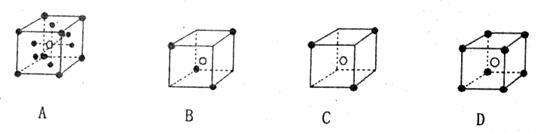
��1����ͼ��ʾһЩ����Ľṹ�������������д����ɱ����� ��
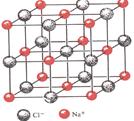
��2��MgO�����ռ乹����NaCl������ͬ��MgO������Mg2������λ��Ϊ________����ͬ��Mg2���Ⱦ��������O2��Χ�ɵĿռ伸�ι�������������������MgO�����۵����NaCl���壬ԭ������������������������ ������������������
��3����C��D����ij��Ԫ���е�����ͬ���칹�壬C��ԭ�ӵ��ӻ������������������������������塣D��ԭ�ӵ��ӻ�����������������D�ܵ����ԭ������ ����
��4��ԭ�Ӿ����ܷ��γ����ܶѻ��ṹ���� ��ԭ���� ��
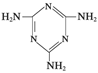
(5)ƫ���ᱵ�����ȶ��Ժã���糣���ߣ���С�ͱ�ѹ������Ͳ���������ж���Ӧ�á�ƫ���ᱵ�����о����Ľṹʾ��ͼ����ͼ�����Ļ�ѧʽ�� ��������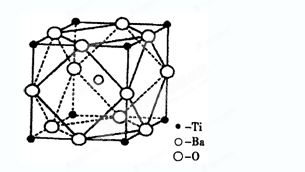
��1����ͼ��ʾһЩ����Ľṹ�������������д����ɱ����� ��

��2��MgO�����ռ乹����NaCl������ͬ��MgO������Mg2������λ��Ϊ________����ͬ��Mg2���Ⱦ��������O2��Χ�ɵĿռ伸�ι�������������������MgO�����۵����NaCl���壬ԭ������������������������ ������������������
��3����C��D����ij��Ԫ���е�����ͬ���칹�壬C��ԭ�ӵ��ӻ������������������������������塣D��ԭ�ӵ��ӻ�����������������D�ܵ����ԭ������ ����
��4��ԭ�Ӿ����ܷ��γ����ܶѻ��ṹ���� ��ԭ���� ��
(5)ƫ���ᱵ�����ȶ��Ժã���糣���ߣ���С�ͱ�ѹ������Ͳ���������ж���Ӧ�á�ƫ���ᱵ�����о����Ľṹʾ��ͼ����ͼ�����Ļ�ѧʽ�� ��������

��10�֣��������պ�1�֣�����ÿ��1�֣���B��
��6���������壻MgO���������ӵĵ��������NaCl�����Ӽ��ƽ������С��NaCl��
��sp3�ӻ���ԭ�ӡ�sp2�ӻ���ÿ��̼ԭ����δ�����ӻ���һ��2p����ϵ����ڲ��������˶���ʯī�������ɵ��ӣ���
�Ȳ��ܣ����ۼ��б���������λ��ԶС��12�� ��BaTiO3
��6���������壻MgO���������ӵĵ��������NaCl�����Ӽ��ƽ������С��NaCl��
��sp3�ӻ���ԭ�ӡ�sp2�ӻ���ÿ��̼ԭ����δ�����ӻ���һ��2p����ϵ����ڲ��������˶���ʯī�������ɵ��ӣ���
�Ȳ��ܣ����ۼ��б���������λ��ԶС��12�� ��BaTiO3
��1���ɱ��γɵľ����Ƿ��Ӿ��壬��ѡB��
��2���Ȼ��Ƶ���λ����6����MgO������Mg2������λ��Ҳ��6����ͬ��Mg2���Ⱦ��������O2����6����Χ�ɵĿռ伸�ι������������塣MgO���������ӵĵ��������NaCl�����Ӽ��ƽ������С��NaCl��������þ�ľ����ܴ����Ȼ��Ƶģ������۵�ߡ�
��3�����߽ṹ��֪��C�ǽ��ʯ��̼ԭ����sp3�ӻ���D��ʯī��̼ԭ����sp2�ӻ�������ÿ��̼ԭ����δ�����ӻ���һ��2p����ϵ����ڲ��������˶�����ʯī�������ɵ��ӣ�������ʯī�ܵ��硣
��4�����ڹ��ۼ��б���������λ��ԶС��12������ԭ�Ӿ��岻���γ����ܶѻ��ṹ��
��5�����ݼӰ�ṹ��֪�����е���ԭ����8��1/8��1����ԭ����12��1/4��3��Ba�����ģ����Ի�ѧʽΪBaTiO3��
��2���Ȼ��Ƶ���λ����6����MgO������Mg2������λ��Ҳ��6����ͬ��Mg2���Ⱦ��������O2����6����Χ�ɵĿռ伸�ι������������塣MgO���������ӵĵ��������NaCl�����Ӽ��ƽ������С��NaCl��������þ�ľ����ܴ����Ȼ��Ƶģ������۵�ߡ�
��3�����߽ṹ��֪��C�ǽ��ʯ��̼ԭ����sp3�ӻ���D��ʯī��̼ԭ����sp2�ӻ�������ÿ��̼ԭ����δ�����ӻ���һ��2p����ϵ����ڲ��������˶�����ʯī�������ɵ��ӣ�������ʯī�ܵ��硣
��4�����ڹ��ۼ��б���������λ��ԶС��12������ԭ�Ӿ��岻���γ����ܶѻ��ṹ��
��5�����ݼӰ�ṹ��֪�����е���ԭ����8��1/8��1����ԭ����12��1/4��3��Ba�����ģ����Ի�ѧʽΪBaTiO3��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �׳ơ����������������������谷����������
�׳ơ����������������������谷����������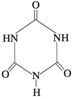 ��
��

 ԭ����Χ���������
ԭ����Χ��������� ԭ���� ����
ԭ���� ����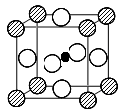
 =1.414��CXO���徧���ṹΪNaCl�ͣ����ھ���ȱ�ݣ�xֵΪ0.88�������߳�Ϊ4.28��10��10m������������Cԭ��֮�����̾���Ϊ___________m����ȷ��0.01�����������е�C�ֱ�ΪC2����C3�����˾�����C2����C3�������������Ϊ_________��
=1.414��CXO���徧���ṹΪNaCl�ͣ����ھ���ȱ�ݣ�xֵΪ0.88�������߳�Ϊ4.28��10��10m������������Cԭ��֮�����̾���Ϊ___________m����ȷ��0.01�����������е�C�ֱ�ΪC2����C3�����˾�����C2����C3�������������Ϊ_________��